Rickettsia parkeri
| Rickettsia parkeri | |
|---|---|
| |
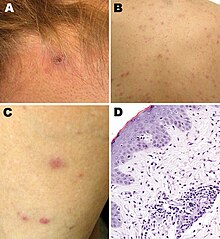
| Rickettsia parkeri rickettsiosis skin lesions: A - eschar after tick bite on neck; B, C - papulovesicular rash on back and leg; D - micrograph of biopsy specimen | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Alphaproteobacteria |
| Order: | Rickettsiales |
| Family: | Rickettsiaceae |
| Genus: | Rickettsia |
| Species group: | Spotted fever group |
| Species: | R. parkeri
|
| Binomial name | |
| Rickettsia parkeri Lackman et al., 1965
| |
Rickettsia parkeri (abbreviated R. parkeri) is a gram-negative intracellular bacterium. The organism is found in the Western Hemisphere and is transmitted via the bite of hard ticks of the genus Amblyomma. R. parkeri causes mild spotted fever disease in humans, whose most common signs and symptoms are fever, an eschar at the site of tick attachment, rash, headache, and muscle aches. Doxycycline is the most common drug used to reduce the symptoms associated with disease.
Biology[edit]
R. parkeri is classified in the spotted fever group of the genus Rickettsia.[1][2] Genetically, its close relatives include R. africae, R. sibirica, R. conorii, R. rickettsii, R. peacockii, and R. honei.[1]
The organism has been isolated from numerous species of ticks in the genus Amblyomma: A. americanum in the United States; A. aureolatum in Brazil; A. maculatum in Mexico, Peru, and the United States; A. nodosum in Brazil; A. ovale in Brazil and Mexico; A. parvitarsum in Argentina and Chile; A. tigrinum in Argentina, Bolivia, Brazil, and Uruguay; and A. triste in Argentina, Brazil, the United States, and Uruguay.[2][3][4][5] Different ticks may carry different strains of the organism. R. parkeri sensu stricto ("in the strict sense") is found in A. maculatum and A. triste; R. parkeri strain NOD, in A. nodosum; R. parkeri strain Parvitarsum, in A. parvitarsum; and R. parkeri strain Atlantic rainforest, in A. aureolatum and A. ovale.[2]
Human infections[edit]
The first report of a confirmed human case of infection with R. parkeri was published in 2004.[6][7] The person was infected in the state of Virginia in the United States.[6] Other confirmed or probable human cases have been reported to have acquired infection elsewhere in the United States (e.g., Arizona, Georgia, and Mississippi), as well as in Argentina, Brazil, Colombia, Mexico, and Uruguay.[8][9] Terms used to describe human infection with R. parkeri include "American boutonneuse fever" because of its similarity to boutonneuse fever caused by Rickettsia conorii;[10] "American tick bite fever" because of its similarity to African tick bite fever caused by Rickettsia africae;[11] "Tidewater spotted fever," after the Tidewater region in the eastern United States;[12] and "Rickettsia parkeri rickettsiosis" or "R. parkeri rickettsiosis."[7][12]
Epidemiology[edit]
Of all human cases documented in the medical literature, 87% were 18-64 years of age, and most cases were male.[8] Brazil, Argentina, and the United States accounted for the majority of cases in the medical literature.[8] In the United States, most of the 40 cases reported to the Centers for Disease Control and Prevention (CDC) as of 2016 became infected between the months of July and September.[13]: 5–6
Diagnosis[edit]
The CDC recommends polymerase chain reaction (PCR) of a biopsy or swab of an eschar, or PCR of a biopsy of a rash, for diagnosis of R. parkeri infection.[13]: 27 In addition, indirect immunofluorescence antibody (IFA) assays using paired acute and convalescent sera can be used.[13]: 27
Clinical manifestations[edit]
A 2008 study compared 12 R. parkeri cases with 208 Rocky Mountain spotted fever cases caused by R. rickettsii.[7] Although both R. parkeri and R. rickettsii caused fever, rash, myalgia, and headache, R. parkeri caused eschars and R. rickettsii did not.[7] Furthermore, the percentage of patients hospitalized was lower for R. parkeri than for R. rickettsii (33% vs 78%), and R. parkeri led to no deaths while R. rickettsii led to death in 7% of cases.[7]
A 2021 systematic review of 32 confirmed and 45 probable cases of human infection with R. parkeri determined that 94% of the confirmed cases had fever, 91% an eschar, 72% a rash, 56% headache, and 56% myalgia, with similar percentages among the probable cases.[8] The rash was most frequently described as papular or macular.[8] Among the confirmed and probable cases, the most common treatment was doxycycline, followed by tetracycline.[8] Although 9% of all the cases were hospitalized, there was a "100% rate of clinical recovery."[8]
History[edit]
In 1939, Ralph R. Parker, director of the Rocky Mountain Laboratory, and others published a paper on "a rickettsia-like infectious agent."[7][14] The agent, found in Amblyomma maculatum ticks collected from cows in Texas, produced mild disease in guinea pigs.[7][14] In 1965, Lackman and others named the rickettsial organism R. parkeri after Parker.[2][15]
References[edit]
- ^ a b Perlman SJ, Hunter MS, Zchori-Fein E (2006). "The emerging diversity of Rickettsia". Proc Biol Sci. 273 (1598): 2097–2106. doi:10.1098/rspb.2006.3541. PMC 1635513. PMID 16901827.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Nieri-Bastos FA, Marcili A, De Sousa R, Paddock CD, Labruna MB (2018). "Phylogenetic evidence for the existence of multiple strains of Rickettsia parkeri in the New World". Appl Environ Microbiol. 84 (8): e02872–17. Bibcode:2018ApEnM..84E2872N. doi:10.1128/AEM.02872-17. PMC 5881050. PMID 29439989.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR; et al. (2009). "Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA". Emerg Infect Dis. 15 (9): 1471–3. doi:10.3201/eid1509.090330. PMC 2819870. PMID 19788817.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Delgado-de la Mora J, Sánchez-Montes S, Licona-Enríquez JD, Delgado-de la Mora D, Paddock CD, Beati L; et al. (2019). "Rickettsia parkeri and Candidatus Rickettsia andeanae in Tick of the Amblyomma maculatum Group, Mexico". Emerg Infect Dis. 25 (4): 836–838. doi:10.3201/eid2504.181507. PMC 6433039. PMID 30882330.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sánchez-Montes S, Ballados-González GG, Hernández-Velasco A, Zazueta-Islas HM, Solis-Cortés M, Miranda-Ortiz H; et al. (2019). "Molecular Confirmation of Rickettsia parkeri in Amblyomma ovale Ticks, Veracruz, Mexico". Emerg Infect Dis. 25 (12): 2315–2317. doi:10.3201/eid2512.190964. PMC 6874242. PMID 31742525.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J; et al. (2004). "Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States". Clin Infect Dis. 38 (6): 805–811. doi:10.1086/381894. PMID 14999622.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC; et al. (2008). "Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever". Clin Infect Dis. 47 (9): 1188–1196. doi:10.1086/592254. PMID 18808353.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Silva-Ramos CR, Hidalgo M, Faccini-Martínez ÁA (2021). "Clinical, epidemiological, and laboratory features of Rickettsia parkeri rickettsiosis: A systematic review". Ticks Tick Borne Dis. 12 (4): 101734. doi:10.1016/j.ttbdis.2021.101734. PMID 33989945. S2CID 234596034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Torres-Castro M, Sánchez-Montes S, Colunga-Salas P, Noh-Pech H, Reyes-Novelo E, Rodríguez-Vivas RI (2022). "Molecular confirmation of Rickettsia parkeri in humans from Southern Mexico". Zoonoses Public Health. 69 (4): 382–386. doi:10.1111/zph.12927. PMID 35142079. S2CID 246701684.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Goddard J, Paddock CD (2005). "Observations on distribution and seasonal activity of the Gulf Coast tick in Mississippi". J Med Entomol. 42 (2): 176–179. doi:10.1093/jmedent/42.2.176. PMID 15799527.
- ^ Iweriebor BC, Nqoro A, Obi CL (2020). "Rickettsia africae an agent of African tick bite fever in ticks collected from domestic animals in Eastern Cape, South Africa". Pathogens. 9 (8): 631. doi:10.3390/pathogens9080631. PMC 7459594. PMID 32748891.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wright CL, Nadolny RM, Jiang J, Richards AL, Sonenshine DE, Gaff HD; et al. (2011). "Rickettsia parkeri in Gulf Coast ticks, southeastern Virginia, USA". Emerg Infect Dis. 17 (5): 896–8. doi:10.3201/eid1705.101836. PMC 3321792. PMID 21529406.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS; et al. (2016). "Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain Spotted Fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States". MMWR Recomm Rep. 65 (2): 1–44. doi:10.15585/mmwr.rr6502a1. PMID 27172113.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Parker RR, Kohls GM, Cox GW, Davis GE (1939). "Observations on an infectious agent from Amblyomma maculatum". Public Health Rep. 54 (32): 1482–1484. doi:10.1086/592254. PMID 18808353.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lackman DB, Bell EJ, Stoenner HG, Pickens EG (1965). "The Rocky Mountain spotted fever group of rickettsias". Health Lab Sci. 2: 135–141. PMID 14318051.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links[edit]
- "Rickettsia parkeri rickettsiosis". Centers for Disease Control and Prevention. January 10, 2019.
