Bemiparin sodium
 | |
| Clinical data | |
|---|---|
| Trade names | Badyket, Ivor, Hibor, Zibor, others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Subcutaneous injection (except for haemodialysis) |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 96% (estimated) |
| Elimination half-life | 5–6 hours |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| Chemical and physical data | |
| Molar mass | 3600 g/mol (average) |
| | |
Bemiparin (trade names Ivor and Zibor, among others) is an antithrombotic and belongs to the group of low molecular weight heparins (LMWH).[1]
Medical uses[edit]
Bemiparin is used for the prevention of thromboembolism after surgery, and to prevent blood clotting in the extracorporeal circuit in haemodialysis.[2]
Contraindications[edit]
The medication is contraindicated in patients with a history of heparin-induced thrombocytopenia with or without disseminated intravascular coagulation; acute bleeding or risk of bleeding; injury or surgery of the central nervous system, eyes or ears; severe liver or pancreas impairment; and acute or subacute bacterial endocarditis.[2]
Interactions[edit]
No interaction studies have been conducted. Drugs that are expected to increase the risk of bleeding in combination with bemiparin include other anticoagulants, aspirin and other NSAIDs, antiplatelet drugs, and corticosteroids.[2]
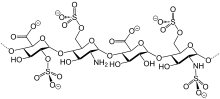
Chemistry[edit]
Like semuloparin, bemiparin is classified as an ultra-LMWH because of its low molecular mass of 3600 g/mol on average.[3] (Enoxaparin has 4500 g/mol.) These heparins have lower anti-thrombin activity than classical LMWHs and act mainly on factor Xa, reducing the risk of bleeding.[4]
References[edit]
- ^ Chapman TM, Goa KL (2003). "Bemiparin: a review of its use in the prevention of venous thromboembolism and treatment of deep vein thrombosis". Drugs. 63 (21): 2357–77. doi:10.2165/00003495-200363210-00009. PMID 14524738. S2CID 195692039.
- ^ a b c Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. 2018. Ivor 2500 IE Anti-Xa/0,2 ml Injektionslösung in Fertigspritzen.
- ^ Planès A (September 2003). "Review of bemiparin sodium--a new second-generation low molecular weight heparin and its applications in venous thromboembolism". Expert Opinion on Pharmacotherapy. 4 (9): 1551–61. doi:10.1517/14656566.4.9.1551. PMID 12943485. S2CID 13566575.
- ^ Jeske WP, Hoppensteadt D, Gray A, Walenga JM, Cunanan J, Myers L, Fareed J, Bayol A, Rigal H, Viskov C (October 2011). "A common standard is inappropriate for determining the potency of ultra low molecular weight heparins such as semuloparin and bemiparin". Thrombosis Research. 128 (4): 361–7. doi:10.1016/j.thromres.2011.03.001. PMID 21458847.
External links[edit]
- bemiparin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
