Lithium borate
| |
| Names | |
|---|---|
| Other names
Lithium borate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.364 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li2B4O7 | |
| Molar mass | 169.11 g/mol |
| Appearance | white powder |
| Density | 2.4 g/cm3, solid |
| Melting point | 917 °C (1,683 °F; 1,190 K) |
| moderately soluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium borate, also known as lithium tetraborate is an inorganic compound with the formula Li2B4O7. A colorless solid, lithium borate is used in making glasses and ceramics.
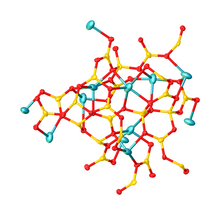
Structure[edit]
Its structure consists of a polymeric borate backbone. The Li+ centers are bound to four and five oxygen ligands. Boron centers are trigonal and tetrahedral.[1][2]

Lithium borate can be used in the laboratory as LB buffer for gel electrophoresis of DNA and RNA. It is also used in the borax fusion method to vitrify mineral powder specimens for analysis by WDXRF spectroscopy.[3]
See also[edit]
- LB buffer
- Lithium metaborate (LiBO2)
References[edit]
- ^ Sennova, N.; Bubnova, R.; Shepelev, Yu.; Filatov, S.; Yakovleva, O. (2007). "Li2B4O7 Crystal structure in anharmonic approximation at 20, 200, 400 and 500 °C". Journal of Alloys and Compounds. 428 (1–2): 290–296. doi:10.1016/j.jallcom.2006.03.049.
- ^ Natalia Sennova. R. S. Bubnova; G. Cordier; B. Albert; S. K. Filatov; L. Isaenko (2008). "Temperature‐dependent Changes of the Crystal Structure of Li2B4O7". Zeitschrift für Anorganische und Allgemeine Chemie. 634 (14): 2601–2607. doi:10.1002/zaac.200800295.
- ^ Ron Jenkins, X-Ray Fluorescence Spectrometry, Second Edition, J. Wiley & Sons Inc., 1999, ISBN 0-471-29942-1, p 146-7.

