Bixin
(Redirected from Norbixin)
| |
| Names | |
|---|---|
| IUPAC name
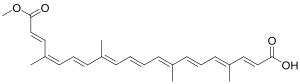
(2E,4E,6E,8E,10E,12E,14E,16Z,18E)-20-Methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18-nonaenoic acid
| |
| Other names
cis-Bixin; α-Bixin; 9-cis-6,6'-Diapo-ψ,ψ-carotenedioic acid, 6-methyl ester
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.499 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H30O4 | |
| Molar mass | 394.511 g·mol−1 |
| Appearance | Orange crystals |
| Melting point | 198 °C (cis-isomer) 217 °C (trans-isomer) |
| Insoluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bixin is an apocarotenoid found in the seeds of the achiote tree (Bixa orellana)[2] from which it derives its name. It is commonly extracted from the seeds to form annatto, a natural food coloring, containing about 5% pigments of which 70–80% are bixin.[3]
Applications[edit]
Several thousand tons are harvested annually.[4]
Chemical properties[edit]
Bixin is unstable. It isomerizes into trans-bixin (β-bixin), the double-bond isomer.[1]
Bixin is soluble in fats and alcohols but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
References[edit]
- ^ a b Merck Index, 11th Edition, 1320
- ^ Bouvier, Florence; Dogbo, Odette; Camara, Bilal (2003). "Biosynthesis of the Food and Cosmetic Plant Pigment Bixin (Annatto)". Science. 300 (5628): 2089–2091. Bibcode:2003Sci...300.2089B. doi:10.1126/science.1085162. ISSN 0036-8075. JSTOR 3834418. PMID 12829782. S2CID 560600.
- ^ Executive Summary Bixin Archived July 21, 2011, at the Wayback Machine, National Toxicology Program
- ^ Stringheta, Paulo C.; Silva, Pollyanna I.; Costa, André G.V. (2018). "Annatto/Urucum— Bixa orellana". Exotic Fruits. pp. 23–30. doi:10.1016/B978-0-12-803138-4.00006-X. ISBN 9780128031384.





