SR-142948
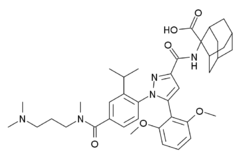 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C39H51N5O6 |
| Molar mass | 685.866 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
SR-142948 is a drug used in scientific research which is a non-peptide antagonist selective for the neurotensin receptors, although not selective between subtypes.[1]
Study[edit]
SR-142948 has been used to study the role of neurotensin in the regulation of dopamine receptor activity[2][3][4][5] and glutamate signalling in the brain.[6][7]
In animal studies, SR-142948 blocked the effects of stimulant drugs,[8] including MDMA.[9]
References[edit]
- ^ Nalivaiko E, Michaud JC, Soubrié P, Le Fur G (October 1998). "Electrophysiological evidence for putative subtypes of neurotensin receptors in guinea-pig mesencephalic dopaminergic neurons". Neuroscience. 86 (3): 799–811. doi:10.1016/S0306-4522(98)00084-0. PMID 9692718. S2CID 41883743.
- ^ Alonso R, Gnanadicom H, Fréchin N, Fournier M, Le Fur G, Soubrié P (March 1999). "Blockade of neurotensin receptors suppresses the dopamine D1/D2 synergism on immediate early gene expression in the rat brain". The European Journal of Neuroscience. 11 (3): 967–74. doi:10.1046/j.1460-9568.1999.00506.x. PMID 10103090. S2CID 9622032.
- ^ Matsuyama S, Higashi H, Maeda H, Greengard P, Nishi A (April 2002). "Neurotensin regulates DARPP-32 thr34 phosphorylation in neostriatal neurons by activation of dopamine D1-type receptors". Journal of Neurochemistry. 81 (2): 325–34. doi:10.1046/j.1471-4159.2002.00822.x. PMID 12064480. S2CID 31073155.
- ^ Leonetti M, Brun P, Sotty F, Steinberg R, Soubrié P, Bert L, et al. (June 2002). "The neurotensin receptor antagonist SR 142948A blocks the efflux of dopamine evoked in nucleus accumbens by neurotensin ejection into the ventral tegmental area". Naunyn-Schmiedeberg's Archives of Pharmacology. 365 (6): 427–33. doi:10.1007/s00210-002-0574-6. PMID 12070755. S2CID 2934039.
- ^ Panayi F, Colussi-Mas J, Lambás-Señas L, Renaud B, Scarna H, Bérod A (May 2005). "Endogenous neurotensin in the ventral tegmental area contributes to amphetamine behavioral sensitization". Neuropsychopharmacology. 30 (5): 871–9. doi:10.1038/sj.npp.1300638. PMID 15637639.
- ^ Matsuyama S, Fukui R, Higashi H, Nishi A (September 2003). "Regulation of DARPP-32 Thr75 phosphorylation by neurotensin in neostriatal neurons: involvement of glutamate signalling". The European Journal of Neuroscience. 18 (5): 1247–53. doi:10.1046/j.1460-9568.2003.02859.x. PMID 12956723. S2CID 23999624.
- ^ Yin HH, Adermark L, Lovinger DM (January 2008). "Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling". Neuropharmacology. 54 (1): 79–86. doi:10.1016/j.neuropharm.2007.06.004. PMC 2697967. PMID 17675102.
- ^ Reynolds SM, Geisler S, Bérod A, Zahm DS (July 2006). "Neurotensin antagonist acutely and robustly attenuates locomotion that accompanies stimulation of a neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area". The European Journal of Neuroscience. 24 (1): 188–96. doi:10.1111/j.1460-9568.2006.04791.x. PMID 16882016. S2CID 35592039.
- ^ Marie-Claire C, Palminteri S, Romualdi P, Noble F (June 2008). "Effects of the selective neurotensin antagonist SR 142948A on 3,4-methylenedioxymethamphetamine-induced behaviours in mice". Neuropharmacology. 54 (7): 1107–11. doi:10.1016/j.neuropharm.2008.03.001. PMID 18410947. S2CID 15455279.
