Energy density
| Energy density | |
|---|---|
| SI unit | J/m3 |
Other units | J/L, W⋅h/L |
| In SI base units | m−1⋅kg⋅s−2 |
Derivations from other quantities | U = E/V |
| Dimension | |
In physics, energy density is the quotient between the amount of energy stored in a given system or contained in a given region of space and the volume of the system or region considered. Often only the useful or extractable energy is measured. It is sometimes confused with stored energy per unit mass, which is called specific energy or gravimetric energy density.
There are different types of energy stored, corresponding to a particular type of reaction. In order of the typical magnitude of the energy stored, examples of reactions are: nuclear, chemical (including electrochemical), electrical, pressure, material deformation or in electromagnetic fields. Nuclear reactions take place in stars and nuclear power plants, both of which derive energy from the binding energy of nuclei. Chemical reactions are used by organisms to derive energy from food and by automobiles from the combustion of gasoline. Liquid hydrocarbons (fuels such as gasoline, diesel and kerosene) are today the densest way known to economically store and transport chemical energy at a large scale (1 kg of diesel fuel burns with the oxygen contained in ≈15 kg of air). Burning local biomass fuels supplies household energy needs (cooking fires, oil lamps, etc.) worldwide. Electrochemical reactions are used by devices such as laptop computers and mobile phones to release energy from batteries.
Energy per unit volume has the same physical units as pressure, and in many situations is synonymous. For example, the energy density of a magnetic field may be expressed as and behaves like a physical pressure. The energy required to compress a gas to a certain volume may be determined by multiplying the difference between the gas pressure and the external pressure by the change in volume. A pressure gradient describes the potential to perform work on the surroundings by converting internal energy to work until equilibrium is reached.
In cosmological and other contexts in general relativity, the energy densities considered relate to the elements of the stress-energy tensor and therefore do include the rest mass energy as well as energy densities associated with pressure.
Chemical energy
[edit]When discussing the chemical energy contained, there are different types which can be quantified depending on the intended purpose. One is the theoretical total amount of thermodynamic work that can be derived from a system, at a given temperature and pressure imposed by the surroundings, called exergy. Another is the theoretical amount of electrical energy that can be derived from reactants that are at room temperature and atmospheric pressure. This is given by the change in standard Gibbs free energy. But as a source of heat or for use in a heat engine, the relevant quantity is the change in standard enthalpy or the heat of combustion.
There are two kinds of heat of combustion:
- The higher value (HHV), or gross heat of combustion, includes all the heat released as the products cool to room temperature and whatever water vapor is present condenses.
- The lower value (LHV), or net heat of combustion, does not include the heat which could be released by condensing water vapor, and may not include the heat released on cooling all the way down to room temperature.
A convenient table of HHV and LHV of some fuels can be found in the references.[1]
In energy storage and fuels
[edit]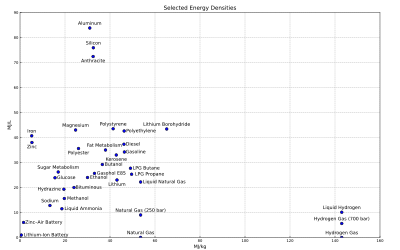
For energy storage, the energy density relates the stored energy to the volume of the storage equipment, e.g. the fuel tank. The higher the energy density of the fuel, the more energy may be stored or transported for the same amount of volume. The energy of a fuel per unit mass is called its specific energy.
The adjacent figure shows the gravimetric and volumetric energy density of some fuels and storage technologies (modified from the Gasoline article). Some values may not be precise because of isomers or other irregularities. The heating values of the fuel describe their specific energies more comprehensively.
The density values for chemical fuels do not include the weight of the oxygen required for combustion. The atomic weights of carbon and oxygen are similar, while hydrogen is much lighter. Figures are presented in this way for those fuels where in practice air would only be drawn in locally to the burner. This explains the apparently lower energy density of materials that contain their own oxidizer (such as gunpowder and TNT), where the mass of the oxidizer in effect adds weight, and absorbs some of the energy of combustion to dissociate and liberate oxygen to continue the reaction. This also explains some apparent anomalies, such as the energy density of a sandwich appearing to be higher than that of a stick of dynamite.
Given the high energy density of gasoline, the exploration of alternative media to store the energy of powering a car, such as hydrogen or battery, is strongly limited by the energy density of the alternative medium. The same mass of lithium-ion storage, for example, would result in a car with only 2% the range of its gasoline counterpart. If sacrificing the range is undesirable, much more storage volume is necessary. Alternative options are discussed for energy storage to increase energy density and decrease charging time, such as supercapacitors.[9][10][11][12]
No single energy storage method boasts the best in specific power, specific energy, and energy density. Peukert's law describes how the amount of useful energy that can be obtained (for a lead-acid cell) depends on how quickly it is pulled out.
Efficiency
[edit]In general an engine will generate less kinetic energy due to inefficiencies and thermodynamic considerations—hence the specific fuel consumption of an engine will always be greater than its rate of production of the kinetic energy of motion.
Energy density differs from energy conversion efficiency (net output per input) or embodied energy (the energy output costs to provide, as harvesting, refining, distributing, and dealing with pollution all use energy). Large scale, intensive energy use impacts and is impacted by climate, waste storage, and environmental consequences.
Nuclear energy
[edit]The greatest energy source by far is matter itself, according to the mass-energy equivalence. This energy is described by E = mc2, where c is the speed of light. In terms of density, m = ρV, where ρ is the mass per unit volume, V is the volume of the mass itself. This energy can be released by the processes of nuclear fission (~0.1%), nuclear fusion (~1%), or the annihilation of some or all of the matter in the volume V by matter-antimatter collisions (100%).[citation needed]
The most effective ways of accessing this energy, aside from antimatter, are fusion and fission. Fusion is the process by which the sun produces energy which will be available for billions of years (in the form of sunlight and heat). However as of 2024, sustained fusion power production continues to be elusive. Power from fission in nuclear power plants (using uranium and thorium) will be available for at least many decades or even centuries because of the plentiful supply of the elements on earth,[13] though the full potential of this source can only be realized through breeder reactors, which are, apart from the BN-600 reactor, not yet used commercially.[14]
Fission reactors
[edit]Nuclear fuels typically have volumetric energy densities at least tens of thousands of times higher than chemical fuels. A 1 inch tall uranium fuel pellet is equivalent to about 1 ton of coal, 120 gallons of crude oil, or 17,000 cubic feet of natural gas.[15] In light-water reactors, 1 kg of natural uranium – following a corresponding enrichment and used for power generation– is equivalent to the energy content of nearly 10,000 kg of mineral oil or 14,000 kg of coal.[16] Comparatively, coal, gas, and petroleum are the current primary energy sources in the U.S.[17] but have a much lower energy density.
The density of thermal energy contained in the core of a light-water reactor (pressurized water reactor (PWR) or boiling water reactor (BWR)) of typically 1 GWe (1,000 MW electrical corresponding to ≈3,000 MW thermal) is in the range of 10 to 100 MW of thermal energy per cubic meter of cooling water depending on the location considered in the system (the core itself (≈30 m3), the reactor pressure vessel (≈50 m3), or the whole primary circuit (≈300 m3)). This represents a considerable density of energy that requires a continuous water flow at high velocity at all times in order to remove heat from the core, even after an emergency shutdown of the reactor.
The incapacity to cool the cores of three BWRs at Fukushima after the 2011 tsunami and the resulting loss of external electrical power and cold source caused the meltdown of the three cores in only a few hours, even though the three reactors were correctly shut down just after the Tōhoku earthquake. This extremely high power density distinguishes nuclear power plants (NPP's) from any thermal power plants (burning coal, fuel or gas) or any chemical plants and explains the large redundancy required to permanently control the neutron reactivity and to remove the residual heat from the core of NPP's.
Antimatter annihilation
[edit]Because antimatter-matter interactions result in complete conversion from the rest mass to radiant energy, the energy density of this reaction depends on the density of the matter and antimatter used. A neutron star would approximate the most dense system capable of matter-antimatter annihilation. A black hole, although denser than a neutron star, does not have an equivalent anti-particle form, but would offer the same 100% conversion rate of mass to energy in the form of Hawking radiation. Even in the case of relatively small black holes (smaller than astronomical objects) the power output would be tremendous.
Electric and magnetic fields
[edit]Electric and magnetic fields can store energy and its density relates to the strength of the fields within a given volume. This (volumetric) energy density is given by
where E is the electric field, B is the magnetic field, and ε and µ are the permittivity and permeability of the surroundings respectively. The solution will be (in SI units) in joules per cubic metre.
In ideal (linear and nondispersive) substances, the energy density (in SI units) is
where D is the electric displacement field and H is the magnetizing field. In the case of absence of magnetic fields, by exploiting Fröhlich's relationships it is also possible to extend these equations to anisotropic and nonlinear dielectrics, as well as to calculate the correlated Helmholtz free energy and entropy densities.[18]
In the context of magnetohydrodynamics, the physics of conductive fluids, the magnetic energy density behaves like an additional pressure that adds to the gas pressure of a plasma.
Pulsed sources
[edit]When a pulsed laser impacts a surface, the radiant exposure, i.e. the energy deposited per unit of surface, may also be called energy density or fluence.[19]
Table of material energy densities
[edit]This article or section appears to contradict itself. (April 2019) |
The following unit conversions may be helpful when considering the data in the tables: 3.6 MJ = 1 kW⋅h ≈ 1.34 hp⋅h. Since 1 J = 10−6 MJ and 1 m3 = 103 L, divide joule/m3 by 109 to get MJ/L = GJ/m3. Divide MJ/L by 3.6 to get kW⋅h/L.
Chemical reactions (oxidation)
[edit]Unless otherwise stated, the values in the following table are lower heating values for perfect combustion, not counting oxidizer mass or volume. When used to produce electricity in a fuel cell or to do work, it is the Gibbs free energy of reaction (ΔG) that sets the theoretical upper limit. If the produced H2O is vapor, this is generally greater than the lower heat of combustion, whereas if the produced H
2O is liquid, it is generally less than the higher heat of combustion. But in the most relevant case of hydrogen, ΔG is 113 MJ/kg if water vapor is produced, and 118 MJ/kg if liquid water is produced, both being less than the lower heat of combustion (120 MJ/kg).[20]
| Material | Specific energy (MJ/kg) |
Energy density (MJ/L) |
Specific energy (W⋅h/kg) |
Energy density (W⋅h/L) |
Comment | |
|---|---|---|---|---|---|---|
| Hydrogen, liquid | 141.86 (HHV) 119.93 (LHV) |
10.044 (HHV) 8.491 (LHV) |
39,405.6 (HHV) 33,313.9 (LHV) |
2,790.0 (HHV) 2,358.6 (LHV) |
Energy figures apply after reheating to 25 °C.[21]
See note above about use in fuel cells. | |
| Hydrogen, gas (681 atm, 69 MPa, 25 °C) | 141.86 (HHV) 119.93 (LHV) |
5.323 (HHV) 4.500 (LHV) |
39,405.6 (HHV) 33,313.9 (LHV) |
1,478.6 (HHV) 1,250.0 (LHV) |
Data from same reference as for liquid hydrogen.[21]
High-pressure tanks weigh much more than the hydrogen they can hold. The hydrogen may be around 5.7% of the total mass,[22] giving just 6.8 MJ per kg total mass for the LHV. See note above about use in fuel cells. | |
| Hydrogen, gas (1 atm or 101.3 kPa, 25 °C) | 141.86 (HHV) 119.93 (LHV) |
0.01188 (HHV) 0.01005 (LHV) |
39,405.6 (HHV) 33,313.9 (LHV) |
3.3 (HHV) 2.8 (LHV) |
[21] | |
| Diborane | 78.2 | 88.4 | 21,722.2 | 24,600 | [23] | |
| Beryllium | 67.6 | 125.1 | 18,777.8 | 34,750.0 | ||
| Lithium borohydride | 65.2 | 43.4 | 18,111.1 | 12,055.6 | ||
| Boron | 58.9 | 137.8 | 16,361.1 | 38,277.8 | [24][better source needed] | |
| Methane (101.3 kPa, 15 °C) | 55.6 | 0.0378 | 15,444.5 | 10.5 | ||
| LNG (NG at −160 °C) | 53.6[25] | 22.2 | 14,888.9 | 6,166.7 | ||
| CNG (NG compressed to 247 atm, 25 MPa ≈ 3,600 psi) | 53.6[25] | 9 | 14,888.9 | 2,500.0 | ||
| Natural gas | 53.6[25] | 0.0364 | 14,888.9 | 10.1 | ||
| LPG propane | 49.6 | 25.3 | 13,777.8 | 7,027.8 | [26] | |
| LPG butane | 49.1 | 27.7 | 13,638.9 | 7,694.5 | [26] | |
| Gasoline (petrol) | 46.4 | 34.2 | 12,888.9 | 9,500.0 | [26] | |
| Polypropylene plastic | 46.4[27] | 41.7 | 12,888.9 | 11,583.3 | ||
| Polyethylene plastic | 46.3[27] | 42.6 | 12,861.1 | 11,833.3 | ||
| Residential heating oil | 46.2 | 37.3 | 12,833.3 | 10,361.1 | [26] | |
| Diesel fuel | 45.6 | 38.6 | 12,666.7 | 10,722.2 | [26] | |
| 100LL Avgas | 44.0[28] | 31.59 | 12,222.2 | 8,775.0 | ||
| Jet fuel (e.g. kerosene) | 43[29][30][31] | 35 | 11,944.4 | 9,722.2 | Aircraft engine | |
| Gasohol E10 (10% ethanol 90% gasoline by volume) | 43.54 | 33.18 | 12,094.5 | 9,216.7 | ||
| Lithium | 43.1 | 23.0 | 11,972.2 | 6,388.9 | ||
| Biodiesel oil (vegetable oil) | 42.20 | 33 | 11,722.2 | 9,166.7 | ||
| DMF (2,5-dimethylfuran) | 42[32] | 37.8 | 11,666.7 | 10,500.0 | [clarification needed] | |
| Paraffin wax | 42[33] | 37.8 | 11,700 | 10,500 | ||
| Crude oil (tonne of oil equivalent) | 41.868 | 37[25] | 11,630 | 10,278 | ||
| Polystyrene plastic | 41.4[27] | 43.5 | 11,500.0 | 12,083.3 | ||
| Body fat | 38 | 35 | 10,555.6 | 9,722.2 | Metabolism in human body (22% efficiency[34]) | |
| Butanol | 36.6 | 29.2 | 10,166.7 | 8,111.1 | ||
| Gasohol E85 (85% ethanol 15% gasoline by volume) | 33.1 | 25.65[citation needed] | 9,194.5 | 7,125.0 | ||
| Graphite | 32.7 | 72.9 | 9,083.3 | 20,250.0 | ||
| Coal, anthracite | 26–33 | 34–43 | 7,222.2–9,166.7 | 9,444.5–11,944.5 | Figures represent perfect combustion not counting oxidizer, but efficiency of conversion to electricity is ≈36%[5] | |
| Silicon | 32.6 | 75.9 | 9,056 | 21,080 | See Table 1 [35] | |
| Aluminium | 31.0 | 83.8 | 8,611.1 | 23,277.8 | ||
| Ethanol | 30 | 24 | 8,333.3 | 6,666.7 | ||
| DME | 31.7 (HHV) 28.4 (LHV) |
21.24 (HHV) 19.03 (LHV) |
8,805.6 (HHV) 7,888.9 (LHV) |
5,900.0 (HHV) 5,286.1 (LHV) |
[36][37] | |
| Polyester plastic | 26.0[27] | 35.6 | 7,222.2 | 9,888.9 | ||
| Magnesium | 24.7 | 43.0 | 6,861.1 | 11,944.5 | ||
| Phosphorus (white) | 24.30 | 44.30 | 6,750 | 12,310 | [38] | |
| Coal, bituminous | 24–35 | 26–49 | 6,666.7–9,722.2 | 7,222.2–13,611.1 | [5] | |
| PET plastic (impure) | 23.5[39] | < ~32.4 | 6,527.8 | < ~9000 | ||
| Methanol | 19.7 | 15.6 | 5,472.2 | 4,333.3 | ||
| Titanium | 19.74 | 88.93 | 5,480 | 24,700 | burned to titanium dioxide | |
| Hydrazine | 19.5 | 19.3 | 5,416.7 | 5,361.1 | burned to nitrogen and water | |
| Liquid ammonia | 18.6 | 11.5 | 5,166.7 | 3,194.5 | burned to nitrogen and water | |
| Potassium | 18.6 | 16.5 | 5,160 | 4,600 | burned to dry potassium oxide | |
| PVC plastic (improper combustion toxic) | 18.0[27] | 25.2 | 5,000.0 | 7,000.0 | [clarification needed] | |
| Wood | 18.0 | 5,000.0 | [40] | |||
| Peat briquette | 17.7 | 4,916.7 | [41] | |||
| Sugars, carbohydrates, and protein | 17 | 26.2 (dextrose) | 4,722.2 | 7,277.8 | Metabolism in human body (22% efficiency[42])[citation needed] | |
| Calcium | 15.9 | 24.6 | 4,416.7 | 6,833.3 | [citation needed] | |
| Glucose | 15.55 | 23.9 | 4,319.5 | 6,638.9 | ||
| Dry cow dung and camel dung | 15.5[43] | 4,305.6 | ||||
| Coal, lignite | 10–20 | 2,777.8–5,555.6 | [citation needed] | |||
| Sodium | 13.3 | 12.8 | 3,694.5 | 3,555.6 | burned to wet sodium hydroxide | |
| Peat | 12.8 | 3,555.6 | ||||
| Nitromethane | 11.3 | 12.85 | 3,138.9 | 3,570 | ||
| Manganese | 9.46 | 68.2 | 2,630 | 18,900 | burned to manganese dioxide | |
| Sulfur | 9.23 | 19.11 | 2,563.9 | 5,308.3 | burned to sulfur dioxide[44] | |
| Sodium | 9.1 | 8.8 | 2,527.8 | 2,444.5 | burned to dry sodium oxide | |
| Household waste | 8.0[45] | 2,222.2 | ||||
| Iron | 7.4 | 57.7 | 2052.9 | 16004.1 | burned to iron(III) oxide[46] | |
| Iron | 6.7 | 52.2 | 1858.3 | 14487.2 | burned to Iron(II,III) oxide[46] | |
| Zinc | 5.3 | 38.0 | 1,472.2 | 10,555.6 | ||
| Teflon plastic | 5.1 | 11.2 | 1,416.7 | 3,111.1 | combustion toxic, but flame retardant | |
| Iron | 4.9 | 38.2 | 1,361.1 | 10,611.1 | burned to iron(II) oxide[46] | |
| Gunpowder | 4.7–11.3[47] | 5.9–12.9 | 1,600–3,580 | |||
| TNT | 4.184 | 6.92 | 1,162 | 1,920 | ||
| Barium | 3.99 | 14.0 | 1,110 | 3,890 | burned to barium dioxide | |
| ANFO | 3.7 | 1,027.8 |
Electrochemical reactions (batteries)
[edit]| Material | Specific energy (MJ/kg) |
Energy density (MJ/L) |
Specific energy (W⋅h/kg) |
Energy density (W⋅h/L) |
Comment |
|---|---|---|---|---|---|
| Zinc-air battery | 1.59[48] | 6.02 | 441.7 | 1,672.2 | Controlled electric discharge |
| Lithium air battery (rechargeable) | 9.0[49] | 2,500.0 | Controlled electric discharge | ||
| Sodium sulfur battery | 0.54–0.86 | 150–240 | |||
| Lithium metal battery | 1.8 | 4.32 | 500 | 1,200 | Controlled electric discharge |
| Lithium-ion battery | 0.36–0.875[52] | 0.9–2.63 | 100.00–243.06 | 250.00–730.56 | Controlled electric discharge |
| Lithium-ion battery with silicon nanowire anodes | 1.566 | 4.32 | 435[53] | 1,200[53] | Controlled electric discharge |
| Alkaline battery | 0.48[54] | 1.3[55] | Controlled electric discharge | ||
| Nickel-metal hydride battery | 0.41[56] | 0.504–1.46[56] | Controlled electric discharge | ||
| Lead-acid battery | 0.17 | 0.56 | 47.2 | 156 | Controlled electric discharge |
| Supercapacitor (EDLC) | 0.01–0.030[57][58][59][60][61][62][63] | 0.006–0.06[57][58][59][60][61][62] | up to 8.57[63] | Controlled electric discharge | |
| Electrolytic capacitor | 0.00001–0.0002[64] | 0.00001–0.001[64][65][66] | Controlled electric discharge |
Common battery formats
[edit]| Storage device | Energy content (Joule) |
Energy content (W⋅h) |
Typical mass (g) |
Typical dimensions (diameter × height in mm) |
Typical volume (mL) | Energy density by volume (MJ/L) |
Energy density by mass (MJ/kg) |
|---|---|---|---|---|---|---|---|
| Alkaline AA battery[67] | 9,360 | 2.6 | 24 | 14.2 × 50 | 7.92 | 1.18 | 0.39 |
| Alkaline C battery[67] | 34,416 | 9.5 | 65 | 26 × 46 | 24.42 | 1.41 | 0.53 |
| NiMH AA battery | 9,072 | 2.5 | 26 | 14.2 × 50 | 7.92 | 1.15 | 0.35 |
| NiMH C battery | 19,440 | 5.4 | 82 | 26 × 46 | 24.42 | 0.80 | 0.24 |
| Lithium-ion 18650 battery | 28,800–46,800 | 8–13 | 44–49[68] | 18 × 65 | 16.54 | 1.74–2.83 | 0.59–1.06 |
Nuclear reactions
[edit]| Material | Specific energy (MJ/kg) |
Energy density (MJ/L) |
Specific energy (W⋅h/kg) |
Energy density (W⋅h/L) |
Comment |
|---|---|---|---|---|---|
| Antimatter | 89,875,517,874 ≈ 90 PJ/kg | Depends on the density of the antimatter's form | 24,965,421,631,578 ≈ 25 TW⋅h/kg | Depends on the density of the antimatter's form | Annihilation, counting both the consumed antimatter mass and ordinary matter mass |
| Hydrogen (fusion) | 639,780,320[69] but at least 2% of this is lost to neutrinos. | Depends on conditions | 177,716,755,600 | Depends on conditions | Reaction 4H→4He |
| Deuterium (fusion) |
571,182,758[70] | Depends on conditions | 158,661,876,600 | Depends on conditions | Proposed fusion scheme for D+D→4He, by combining D+D→T+H, T+D→4He+n, n+H→D and D+D→3He+n, 3He+D→4He+H, n+H→D |
| Deuterium+tritium (fusion) | 337,387,388[69] | Depends on conditions | 93,718,718,800 | Depends on conditions | D + T → 4He + n Being developed. |
| Lithium-6 deuteride (fusion) | 268,848,415[69] | Depends on conditions | 74,680,115,100 | Depends on conditions | 6LiD → 24He Used in weapons. |
| Plutonium-239 | 83,610,000 | 1,300,000,000–1,700,000,000 (Depends on crystallographic phase) | 23,222,915,000 | 370,000,000,000–460,000,000,000 (Depends on crystallographic phase) | Heat produced in Fission reactor |
| Plutonium-239 | 31,000,000 | 490,000,000–620,000,000 (Depends on crystallographic phase) | 8,700,000,000 | 140,000,000,000–170,000,000,000 (Depends on crystallographic phase) | Electricity produced in Fission reactor |
| Uranium | 80,620,000[71] | 1,539,842,000 | 22,394,000,000 | Heat produced in breeder reactor | |
| Thorium | 79,420,000[71] | 929,214,000 | 22,061,000,000 | Heat produced in breeder reactor (Experimental) | |
| Plutonium-238 | 2,239,000 | 43,277,631 | 621,900,000 | Radioisotope thermoelectric generator. The heat is only produced at a rate of 0.57 W/g. |
In material deformation
[edit]The mechanical energy storage capacity, or resilience, of a Hookean material when it is deformed to the point of failure can be computed by calculating tensile strength times the maximum elongation dividing by two. The maximum elongation of a Hookean material can be computed by dividing stiffness of that material by its ultimate tensile strength. The following table lists these values computed using the Young's modulus as measure of stiffness:
| Material | Energy density by mass
(J/kg) |
Resilience: Energy density by volume
(J/L) |
Density
(kg/L) |
Young's modulus
(GPa) |
Tensile yield strength
(MPa) |
|---|---|---|---|---|---|
| Rubber band | 1,651–6,605[72] | 2,200–8,900[72] | 1.35[72] | ||
| Steel, ASTM A228 (yield, 1 mm diameter) | 1,440–1,770 | 11,200–13,800 | 7.80[73] | 210[73] | 2,170–2,410[73] |
| Acetals | 908 | 754 | 0.831[74] | 2.8[75] | 65 (ultimate)[75] |
| Nylon-6 | 233–1,870 | 253–2,030 | 1.084 | 2–4[75] | 45–90 (ultimate)[75] |
| Copper Beryllium 25-1/2 HT (yield) | 684 | 5,720[76] | 8.36[77] | 131[76] | 1,224[76] |
| Polycarbonates | 433–615 | 520–740 | 1.2[78] | 2.6[75] | 52–62 (ultimate)[75] |
| ABS plastics | 241–534 | 258–571 | 1.07 | 1.4–3.1[75] | 40 (ultimate)[75] |
| Acrylic | 1,530 | 3.2[75] | 70 (ultimate)[75] | ||
| Aluminium 7077-T8 (yield) | 399 | 1,120[76] | 2.81[79] | 71.0[76] | 400[76] |
| Steel, stainless, 301-H (yield) | 301 | 2,410[76] | 8.0[80] | 193[76] | 965[76] |
| Aluminium 6061-T6 (yield @ 24 °C) | 205 | 553 | 2.70[81] | 68.9[81] | 276[81] |
| Epoxy resins | 113–1,810 | 2–3[75] | 26–85 (ultimate)[75] | ||
| Douglas fir Wood | 158–200 | 96 | .481–.609[82] | 13[75] | 50 (compression)[75] |
| Steel, Mild AISI 1018 | 42.4 | 334 | 7.87[83] | 205[83] | 370 (440 Ultimate)[83] |
| Aluminium (not alloyed) | 32.5 | 87.7 | 2.70[84] | 69[75] | 110 (ultimate)[75] |
| Pine (American Eastern White, flexural) | 31.8–32.8 | 11.1–11.5 | .350[85] | 8.30–8.56 (flexural)[85] | 41.4 (flexural)[85] |
| Brass | 28.6–36.5 | 250–306 | 8.4–8.73[86] | 102–125[75] | 250 (ultimate)[75] |
| Copper | 23.1 | 207 | 8.93[86] | 117[75] | 220 (ultimate)[75] |
| Glass | 5.56–10.0 | 13.9–25.0 | 2.5[87] | 50–90[75] | 50 (compression)[75] |
Other release mechanisms
[edit]| Material | Specific energy (MJ/kg) |
Energy density (MJ/L) |
Specific energy (W⋅h/kg) |
Energy density (W⋅h/L) |
Comment |
|---|---|---|---|---|---|
| Silicon (phase change) | 1.790 | 4.5 | 500 | 1,285 | Energy stored through solid to liquid phase change of silicon[88] |
| Strontium bromide hydrate | 0.814 [89] | 1.93 | 628 | Thermal energy of phase change at 88.6 °C (361.8 K) | |
| Liquid nitrogen | 0.77[90] | 0.62 | 213.9 | 172.2 | Maximum reversible work at 77.4 K with 300 K reservoir |
| Compressed air at 30 MPa (4,400 psi) | 0.5 | 0.2 | 138.9 | 55.6 | Potential energy |
| Latent heat of fusion of ice (thermal) | 0.334 | 0.334 | 93.1 | 93.1 | |
| Flywheel | 0.36–0.5 | 5.3 | Kinetic energy | ||
| Water at 100 m dam height | 0.000981 | 0.000978 | 0.272 | 0.272 | Figures represent potential energy, but efficiency of conversion to electricity is 85–90%[91][92] |
See also
[edit]Footnotes
[edit]- ^ "Fossil and Alternative Fuels - Energy Content (2008)". Engineering ToolBox. Retrieved 2018-10-08.
- ^ Jeong, Goojin; Kim, Hansu; Park, Jong Hwan; Jeon, Jaehwan; Jin, Xing; Song, Juhye; Kim, Bo-Ram; Park, Min-Sik; Kim, Ji Man; Kim, Young-Jun (2015). "Nanotechnology enabled rechargeable Li–SO2 batteries: Another approach towards post-lithium-ion battery systems". Energy & Environmental Science. 8 (11): 3173–3180. doi:10.1039/C5EE01659B.
- ^ "Panasonic Develops New Higher-Capacity 18650 Li-Ion Cells." Green Car Congress. N.p., 25 Dec. 2009. Web.
- ^ Stura, Enrico; Nicolini, Claudio (2006). "New nanomaterials for light weight lithium batteries". Analytica Chimica Acta. 568 (1–2): 57–64. Bibcode:2006AcAC..568...57S. doi:10.1016/j.aca.2005.11.025. PMID 17761246.
- ^ a b c Fisher, Julia (2003). Elert, Glenn (ed.). "Energy density of coal". The Physics Factbook. Retrieved 2019-07-28.
- ^ "Heat Values of Various Fuels - World Nuclear Association." World Nuclear Association. N.p., Sept. 2016. Web.
- ^ "Overview of Storage Development DOE Hydrogen Program." Office of Energy Efficiency & Renewable Energy. N.p., May 2000. Web.
- ^ Wong, Kaufui; Dia, Sarah (2017). "Nanotechnology in Batteries". Journal of Energy Resources Technology. 139. doi:10.1115/1.4034860.
- ^ Ionescu-Zanetti, C.; et., al. (2005). "Nanogap capacitors: Sensitivity to sample permittivity changes". Journal of Applied Physics. 99 (2): 024305–024305–5. Bibcode:2006JAP....99b4305I. doi:10.1063/1.2161818. S2CID 120910476.
- ^ Naoi, K.; et., al. (2013). "New generation "nanohybrid supercapacitor"". Accounts of Chemical Research. 46 (5): 1075–1083. doi:10.1021/ar200308h. PMID 22433167.
- ^ Hubler, A.; Osuagwu, O. (2010). "Digital quantum batteries: Energy and information storage in nanovacuum tube arrays". Complexity. 15 (5): NA. doi:10.1002/cplx.20306. S2CID 6994736.
- ^ Lyon, D.; et., al. (2013). "Gap size dependence of the dielectric strength in nano vacuum gaps". IEEE Transactions on Dielectrics and Electrical Insulation. 2 (4): 1467–1471. doi:10.1109/TDEI.2013.6571470. S2CID 709782.
- ^ "Supply of Uranium". world-nuclear.org. 2014-10-08. Archived from the original on 2015-10-17. Retrieved 2015-06-13.
- ^ "Facts from Cohen". Formal.stanford.edu. 2007-01-26. Archived from the original on 2007-04-10. Retrieved 2010-05-07.
- ^ Venditti, Bruno; Content, Sponsored (2021-08-27). "The Power of a Uranium Pellet". Elements by Visual Capitalist. Retrieved 2024-08-11.
- ^ "Fuel comparison". ENS. 2019-05-22. Retrieved 2024-08-11.
- ^ "U.S. Energy Information Administration (EIA) - Annual Energy Review". Eia.doe.gov. 2009-06-26. Archived from the original on 2010-05-06. Retrieved 2010-05-07.
- ^ Parravicini, J. (2018). "Thermodynamic potentials in anisotropic and nonlinear dielectrics". Physica B. 541: 54–60. Bibcode:2018PhyB..541...54P. doi:10.1016/j.physb.2018.04.029. S2CID 125817506.
- ^ "Terminology". Regenerative Laser Therapy.
- ^ CRC Handbook of Chemistry and Physics, 49th Edition, page D-42.
- ^ a b c College of the Desert, “Module 1, Hydrogen Properties”, Revision 0, December 2001 Hydrogen Properties. Retrieved 2014-06-08.
- ^ Mike Millikin (2014-11-18). "Toyota FCV Mirai launches in LA; initial TFCS specs; $57,500 or $499 lease; leaning on Prius analogy". Green Car Congress. Retrieved 2014-11-23.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997), Chemistry of the Elements (2nd ed) (page 164)
- ^ "Boron: A Better Energy Carrier than Hydrogen? (28 February 2009)". Eagle.ca. Retrieved 2010-05-07.
- ^ a b c d Envestra Limited. Natural Gas Archived 2008-10-10 at the Wayback Machine. Retrieved 2008-10-05.
- ^ a b c d e IOR Energy. List of common conversion factors (Engineering conversion factors). Retrieved 2008-10-05.
- ^ a b c d e Paul A. Kittle, Ph.D. "Alternate daily cover materials and subtitle D – The selection technique" (PDF). Archived from the original (PDF) on 2008-05-27. Retrieved 2012-01-25.
- ^ "537.pdf" (PDF). June 1993. Archived from the original (PDF) on 2011-09-29. Retrieved 2012-01-25.
- ^ Gofman, Evelyn (2003). Elert, Glenn (ed.). "Energy density of aviation fuel". The Physics Factbook. Retrieved 2019-07-28.
- ^ "Handbook of Products" (PDF). Air BP. pp. 11–13. Archived from the original (PDF) on 2011-06-08.
- ^ Characteristics of Petroleum Products Stored and Dispensed (PDF), Petroleum Products Division - GN, p. 132, archived from the original (PDF) on 16 January 2017, retrieved 15 January 2017
- ^ Román-Leshkov, Yuriy; Barrett, Christopher J.; Liu, Zhen Y.; Dumesic, James A. (21 June 2007). "Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates". Nature. 447 (7147): 982–985. Bibcode:2007Natur.447..982R. doi:10.1038/nature05923. PMID 17581580. S2CID 4366510.
- ^ Wiener, Harry (January 1947). "Structural Determination of Paraffin Boiling Points". Journal of the American Chemical Society. 69 (1): 17–20. doi:10.1021/ja01193a005. ISSN 0002-7863. PMID 20291038.
- ^ Justin Lemire-Elmore (2004-04-13). "The Energy Cost of Electric and Human-Powered Bicycles" (PDF). p. 5. Retrieved 2009-02-26.
properly trained athlete will have efficiencies of 22 to 26%
- ^ "Silicon as an intermediary between renewable energy and hydrogen" (PDF). Deutsche Bank Research. p. 5. Archived from the original (PDF) on 2008-11-16. Retrieved 16 November 2016.
- ^ Bossel, Ulf (July 2003). "The Physics of the Hydrogen Economy" (PDF). European Fuel Cell News. Archived from the original (PDF) on 2006-03-19. Retrieved 2019-04-06.
The Higher Heating Values are 22.7, 29.7 or 31.7 MJ/kg for methanol, ethanol and DME, respectively, while gasoline contains about 45 MJ per kg.
- ^ "Dimethyl Ether (DME)" (PDF). European Biofuels Technology Platform. 2013-11-18. Retrieved 2019-04-06. DME density and lower heating value were obtained from the table on the first page.
- ^ Green Don; Perry Robert (2008). Perry's chemical engineers' handbook (8th ed.). New York: McGraw-Hill. ISBN 9780071422949.
- ^ "Elite_bloc.indd" (PDF). Archived from the original (PDF) on 2011-07-15. Retrieved 2010-05-07.
- ^ "Biomass Energy Foundation: Fuel Densities". Woodgas.com. Archived from the original on 2010-01-10. Retrieved 2010-05-07.
- ^ "Bord na Mona, Peat for Energy" (PDF). Bnm.ie. Archived from the original (PDF) on 2007-11-19. Retrieved 2012-01-25.
- ^ Justin Lemire-elmore (April 13, 2004). "The Energy Cost of Electric and Human-Powered Bicycle" (PDF). Retrieved 2012-01-25.
- ^ "energy buffers". Home.hccnet.nl. Retrieved 2010-05-07.
- ^ Anne Wignall and Terry Wales. Chemistry 12 Workbook, page 138 Archived 2011-09-13 at the Wayback Machine. Pearson Education NZ ISBN 978-0-582-54974-6
- ^ David E. Dirkse. energy buffers. "household waste 8..11 MJ/kg"
- ^ a b c Thomas C. Allison. (2013). NIST-JANAF Thermochemical Tables - SRD 13 (1.0.2) [dataset]. National Institute of Standards and Technology. https://doi.org/10.18434/T42S31
- ^ Lu, Gui-e; Chang, Wen-ping; Jiang, Jin-yong; Du, Shi-guo (May 2011). "Study on the energy density of gunpowder heat source". 2011 International Conference on Materials for Renewable Energy & Environment. IEEE. pp. 1185–1187. doi:10.1109/ICMREE.2011.5930549. ISBN 978-1-61284-749-8. S2CID 36130191.
- ^ "Technical bulletin on Zinc-air batteries". Duracell. Archived from the original on 2009-01-27. Retrieved 2009-04-21.
- ^ Mitchell, Robert R.; Gallant, Betar M.; Thompson, Carl V.; Shao-Horn, Yang (2011). "All-carbon-nanofiber electrodes for high-energy rechargeable Li–O2 batteries". Energy & Environmental Science. 4 (8): 2952–2958. doi:10.1039/C1EE01496J. S2CID 96799565.
- ^ "Overview of lithium ion batteries" (PDF). Panasonic. Jan 2007. Archived (PDF) from the original on November 7, 2011.
- ^ "Panasonic NCR18650B" (PDF). Archived from the original (PDF) on 2015-07-22.
- ^ [50][51]
- ^ a b "Amprius' silicon nanowire Li-ion batteries power Airbus Zephyr S HAPS solar aircraft". Green Car Congress. Retrieved 2022-12-31.
- ^ "Test of Duracell Ultra Power AA". lygte-info.dk. Retrieved 2019-02-16.
- ^ "Energizer EN91 AA alkaline battery datasheet" (PDF). Retrieved 2016-01-10.
- ^ a b "Test of GP ReCyko+ AA 2700mAh (Green)". lygte-info.dk. Retrieved 2019-02-16.
- ^ a b "Maxwell supercapacitor comparison" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2016-01-10.
- ^ a b "Nesscap ESHSP series supercapacitor datasheet" (PDF). Archived from the original (PDF) on 2016-03-29. Retrieved 2016-01-10.
- ^ a b "Cooper PowerStor XL60 series supercapacitor datasheet" (PDF). Archived from the original (PDF) on 2016-04-02. Retrieved 2016-01-10.
- ^ a b "Kemet S301 series supercapacitor datasheet" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2016-01-10.
- ^ a b "Nichicon JJD series supercapatcitor datasheet" (PDF). Retrieved 2016-01-10.
- ^ a b "skelcap High Energy Ultracapacitor" (PDF). Skeleton Technologies. Archived from the original (PDF) on 2 April 2016. Retrieved 13 October 2015.
- ^ a b "3.0V 3400F Ultracapacitor cell datasheet BCAP3400 P300 K04/05" (PDF). Archived from the original (PDF) on 2020-11-01. Retrieved 2020-01-12.
- ^ a b "Vishay STE series tantalum capacitors datasheet" (PDF). Retrieved 2016-01-10.
- ^ "nichicon TVX aluminum electrolytic capacitors datasheet" (PDF). Retrieved 2016-01-10.
- ^ "nichicon LGU aluminum electrolytic capacitors datasheet" (PDF). Retrieved 2016-01-10.
- ^ a b "Battery Energy Tables". Archived from the original on 2011-12-04.
- ^ "18650 Battery capacities".
- ^ a b c Calculated from fractional mass loss times c squared.
- ^ Calculated from fractional mass loss times c squared. Ball, Justin (2019). "Maximizing specific energy by breeding deuterium". Nuclear Fusion. 59 (10): 106043. arXiv:1908.00834. Bibcode:2019NucFu..59j6043B. doi:10.1088/1741-4326/ab394c. S2CID 199405246.
- ^ a b "Computing the energy density of nuclear fuel". whatisnuclear.com. Retrieved 2014-04-17.
- ^ a b c "How Much Energy Can You Store in a Rubber Band?". Wired. ISSN 1059-1028. Retrieved 2020-01-21.
- ^ a b c "MatWeb - The Online Materials Information Resource". www.matweb.com. Retrieved 2019-12-15.
- ^ PubChem. "Acetal". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-12-12.
- ^ a b c d e f g h i j k l m n o p q r s t u v "Young's Modulus - Tensile and Yield Strength for common Materials". www.engineeringtoolbox.com. Retrieved 2019-12-12.
- ^ a b c d e f g h i Brush Wellman Alloy Products. "Elastic Resilience" (PDF). Technical Tidbits. Retrieved December 15, 2019.
- ^ "C17200 Alloy Specifications | E. Jordan Brookes Company". www.ejbmetals.com. Retrieved 2019-12-15.
- ^ "polycarbonate information and properties". www.polymerprocessing.com. Retrieved 2019-12-12.
- ^ "ASM Material Data Sheet". asm.matweb.com. Retrieved 2019-12-15.
- ^ Sutherland, Karen; Martin, Monica (2004). Elert, Glenn (ed.). "Density of steel". The Physics Factbook. Retrieved 2020-06-18.
- ^ a b c "Aluminum 6061-T6; 6061-T651". www.matweb.com. Retrieved 2021-06-13.
- ^ "Wood Species - Moisture Content and Weight". www.engineeringtoolbox.com. Retrieved 2019-12-12.
- ^ a b c "AISI 1018 Mild/Low Carbon Steel". AZoM.com. 2012-06-28. Retrieved 2020-01-22.
- ^ "ASM Material Data Sheet". asm.matweb.com. Retrieved 2019-12-12.
- ^ a b c "American Eastern White Pine Wood". www.matweb.com. Retrieved 2019-12-15.
- ^ a b "Mass, Weight, Density or Specific Gravity of Different Metals". www.simetric.co.uk. Retrieved 2019-12-12.
- ^ "Physical properties of glass | Saint Gobain Building Glass UK". uk.saint-gobain-building-glass.com. Retrieved 2019-12-12.
- ^ Meroueh, Laureen; Chen, Gang (2020). "Thermal energy storage radiatively coupled to a supercritical Rankine cycle for electric grid support". Renewable Energy. 145: 604–621. doi:10.1016/j.renene.2019.06.036. S2CID 197448761.
- ^ A. Fopah-Lele, J. G. Tamba "A review on the use of SrBr2·6H2O as a potential material for low temperature energy storage systems and building applications", Solar Energy Materials and Solar Cells 164 175-84 (2017).
- ^ C. Knowlen, A.T. Mattick, A.P. Bruckner and A. Hertzberg, "High Efficiency Conversion Systems for Liquid Nitrogen Automobiles", Society of Automotive Engineers Inc, 1988.
- ^ "Hydroelectric Power Generation". www.mpoweruk.com. Woodbank Communications Ltd. Retrieved 13 April 2018.
- ^ "2.1 Power, discharge, head relationship | River Engineering & Restoration at OSU | Oregon State University". rivers.bee.oregonstate.edu. Archived from the original on 14 April 2018. Retrieved 13 April 2018.
Let ε = 0.85, signifying an 85% efficiency rating, typical of an older powerplant.
Further reading
[edit]- The Inflationary Universe: The Quest for a New Theory of Cosmic Origins by Alan H. Guth (1998) ISBN 0-201-32840-2
- Cosmological Inflation and Large-Scale Structure by Andrew R. Liddle, David H. Lyth (2000) ISBN 0-521-57598-2
- Richard Becker, "Electromagnetic Fields and Interactions", Dover Publications Inc., 1964
External links
[edit]- ^ "Aircraft Fuels." Energy, Technology and the Environment Ed. Attilio Bisio. Vol. 1. New York: John Wiley and Sons, Inc., 1995. 257–259
- "Fuels of the Future for Cars and Trucks" – Dr. James J. Eberhardt – Energy Efficiency and Renewable Energy, U.S. Department of Energy – 2002 Diesel Engine Emissions Reduction (DEER) Workshop San Diego, California - August 25–29, 2002
- "Heat values of various fuels – World Nuclear Association". www.world-nuclear.org. Retrieved 4 November 2018.
- "Energy and Energy Types – Springer" (PDF). Retrieved 4 November 2018.



