Phenylacetone

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Phenylpropan-2-one | |
| Other names
Benzyl methyl ketone; Methyl benzyl ketone; Phenyl-2-propanone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.859 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.178 g·mol−1 |
| Appearance | Colorless, pleasant odor |
| Density | 1.006 g/mL |
| Melting point | −15 °C (5 °F; 258 K) |
| Boiling point | 214 to 216 °C (417 to 421 °F; 487 to 489 K) |
| -83.44·10−6 cm3/mol | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phenylacetone, also known as phenyl-2-propanone, is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. It is a mono-substituted benzene derivative, consisting of an acetone attached to a phenyl group. As such, its systematic IUPAC name is 1-phenyl-2-propanone.
This substance is used in the manufacture of methamphetamine and amphetamine, where it is commonly known as P2P.[2][3] Due to illicit drug labs using phenylacetone to make amphetamines, phenylacetone was declared a schedule II controlled substance in the United States in 1980.[4] In humans, phenylacetone occurs as a metabolite of amphetamine and methamphetamine via FMO3-mediated oxidative deamination.[5]
Synthesis
[edit]There are many routes to synthesize phenylacetone. Industry uses the gas-phase ketonic decarboxylation of phenylacetic acid using acetic acid over a ceria-alumina solid acid catalyst.[6] A related laboratory-scale reaction has been described.[7]
An alternative route is zeolite-catalyzed isomerization of phenylpropylene oxide. Another laboratory synthesis involves conventional routes including the Friedel-Crafts alkylation reaction of chloroacetone with benzene in the presence of aluminum chloride catalyst.[8]
Amphetamine metabolism
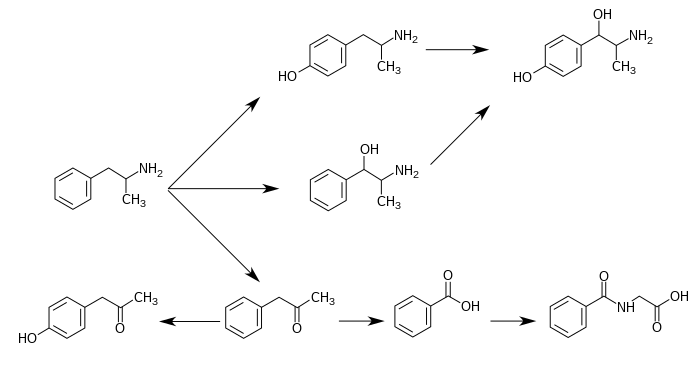
[edit]Metabolic pathways of amphetamine in humans[sources 1]
|
Phenylacetone is an intermediate in the biodegradation of amphetamine. In the human liver, flavin-containing monooxygenase 3 (FMO3) deaminates amphetamines into phenylacetone, which is non-toxic to humans.[20] Phenylacetone is oxidized to benzoic acid, which is converted to hippuric acid by glycine N-acyltransferase (GLYAT) enzymes prior to excretion.
Phenylacetone can undergo para-hydroxylation to 4-hydroxyphenylacetone, which occurs as a metabolite of amphetamine in the human body.
Regulation and culture
[edit]To prevent illicit synthesis of amphetamines from phenylacetone, the precursor phenylacetic acid is subject to regulation in the United States under the Chemical Diversion and Trafficking Act.
In the TV series Breaking Bad, Walter White manufactures methamphetamine using phenylacetone and methylamine through a reductive amination reaction. White produced phenylacetone in a tube furnace using phenylacetic acid and acetic acid. [citation needed]

See also
[edit]- MDP2P – related compound with a methylenedioxy group, and a precursor to MDMA.
- Cyclohexylacetone – the cyclohexane derivative of phenylacetone
- Phenylacetones
- Methamphetamine
- Controlled Substances Act
Notes
[edit]- ^ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[10][15] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[15][17] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[18][19]
References
[edit]- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 15 August 2023.
- ^ Toske, Steven G.; Brown, Jaclyn L.; Miller, Erin E.; Phillips, Monica Z.; Kerr, Susan C.; Hays, Patrick A. (1 May 2019). "Recent methamphetamine profiling trends: Tracking the nitrostyrene method used for P2P production". Forensic Chemistry. 13: 100140. doi:10.1016/j.forc.2018.12.003. ISSN 2468-1709. S2CID 104413528.
- ^ "Synthesis of Phenyl-2-Propanone (P2P) - [www.rhodium.ws]". erowid.org. Retrieved 15 April 2023.
- ^ "Schedules of Controlled Substances; Schedule II Placement of Phenylacetone; (Phenyl-2-propanone, P2P, methyl benzyl ketone, benzyl methyl ketone)" (PDF). Isomer Design. Drug Enforcement Administration. 11 February 1980. Archived (PDF) from the original on 3 March 2022. Retrieved 16 January 2023.
- ^ Cashman, John R.; Xiong, Yeng N.; Xu, Lifen; Janowsky, Aaron (1 March 1999). "N-Oxygenation of Amphetamine and Methamphetamine by the Human Flavin-Containing Monooxygenase (Form 3): Role in Bioactivation and Detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. ISSN 0022-3565. PMID 10027866.
- ^ Siegel, Hardo; Eggersdorfer, Manfred (2000). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
- ^ Hurd, Charles D.; Thomas, Charles L. (July 1936). "Preparation of Dibenzyl Ketone and Phenylacetone". Journal of the American Chemical Society. 58 (7): 1240. doi:10.1021/ja01298a043. ISSN 0002-7863.
- ^ J. Philip Mason; Lewis I. Terry (1 June 1940). "Preparation of Phenylacetone". J. Am. Chem. Soc. 62 (6): 1622. doi:10.1021/ja01863a506. S2CID 101303942.
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ^ a b Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ a b c Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20 (2): 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- ^ Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932. S2CID 23738007.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- ^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201. S2CID 28641000.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- ^ Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - ^ Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- ^ Krueger, Sharon K.; Williams, David E. (1 June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. ISSN 0163-7258. PMC 1828602. PMID 15922018.