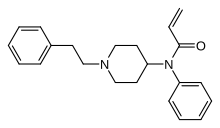
Acrylfentanyl
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H26N2O |
| Molar mass | 334.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Acrylfentanyl (also known as acryloylfentanyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug.[1][2][3][4][5] In animal studies the IC50 (the half maximal inhibitory concentration for acrylfentanyl to displace naloxone) is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.[6][7][8][9]
Side effects
[edit]Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[10]
As acrylamide derivatives are often used in drug discovery to make covalent inhibitors which will bind irreversibly to its target, acrylfentanyl is claimed to be naloxone resistant.[11] However, acute intoxications with acrylfentanyl on mice[12] threatened by naloxone (2 mg/kg) have shown that acrylfentanyl could be displaced from the opioid receptor.
Acrylfentanyl has been linked to 20 deaths in Sweden as well as two deaths in Denmark in summer 2016.[13][14]
Legal status
[edit]Acrylfentanyl is a Schedule I controlled substance in the United States.[15] As of 16 August 2016, it is an illegal medicine in Sweden.[16]
See also
[edit]- 3-Methylbutyrfentanyl
- 4-Fluorobutyrfentanyl
- 4-Methoxybutyrfentanyl
- Acetylfentanyl
- Butyrfentanyl
- Furanylfentanyl
- List of fentanyl analogues
References
[edit]- ^ "Acrylfentanyl (hydrochloride)". Cayman Chemical.
- ^ Ujváry I, Jorge R, Christie R, Le Ruez T, Danielsson HV, Kronstrand R, Elliott S, Gallegos A, Sedefov R, Evans-Brown M (July 2017). "Acryloylfentanyl, a recently emerged new psychoactive substance: a comprehensive review". Forensic Toxicology. 35 (2): 232–243. doi:10.1007/s11419-017-0367-8. S2CID 30184205.
- ^ Breindahl T, Kimergård A, Andreasen MF, Pedersen DS (March 2017). "Identification of a new psychoactive substance in seized material: the synthetic opioid N-phenyl-N-[1-(2-phenethyl)piperidin-4-yl]prop-2-enamide (Acrylfentanyl)". Drug Testing and Analysis. 9 (3): 415–422. doi:10.1002/dta.2046. PMC 5396312. PMID 27476446.
- ^ Helander A, Bäckberg M (January 2017). "New Psychoactive Substances (NPS) - the Hydra monster of recreational drugs". Clinical Toxicology. 55 (1): 1–3. doi:10.1080/15563650.2016.1217003. PMID 27549399. S2CID 35645218.
- ^ Bilel S, Azevedo Neto J, Arfè R, Tirri M, Gaudio RM, Fantinati A, et al. (May 2022). "In vitro and in vivo pharmaco-dynamic study of the novel fentanyl derivatives: Acrylfentanyl, Ocfentanyl and Furanylfentanyl". Neuropharmacology. 209: 109020. doi:10.1016/j.neuropharm.2022.109020. hdl:11392/2497907. PMID 35247453. S2CID 247172069.
- ^ Maryanoff BE, Simon EJ, Gioannini T, Gorissen H (August 1982). "Potential affinity labels for the opiate receptor based on fentanyl and related compounds". Journal of Medicinal Chemistry. 25 (8): 913–919. doi:10.1021/jm00350a006. PMID 6288945.
- ^ Dong N, Lu WC, Chen NY, Zhu YC, Chen KX (January 2005). "Using support vector classification for SAR of fentanyl derivatives". Acta Pharmacologica Sinica. 26 (1): 107–112. doi:10.1111/j.1745-7254.2005.00014.x. PMID 15659122.
- ^ Essawi MY (April 1999). "Fentanyl analogues with a modified propanamido group as potential affinity labels: synthesis and in vivo activity". Die Pharmazie. 54 (4): 307–308. PMID 10234745.
- ^ Zhu YQ, Ge GL, Fang SN, Zhu YC, Dai QY, Tan ZY, et al. (March 1981). "[Studies on potent analgesics. I. Synthesis and analgesic activity of derivatives of fentanyl (author's transl)]". Yao Xue Xue Bao = Acta Pharmaceutica Sinica (in Chinese). 16 (3): 199–210. PMID 6264735.
- ^ Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy. 26 (7): 626–631. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- ^ "PHDMC - Community Overdose Action Team Issues Alert". www.phdmc.org. 1 February 2017.
- ^ Maryanoff BE, Simon EJ, Gioannini T, Gorissen H (August 1982). "Potential affinity labels for the opiate receptor based on fentanyl and related compounds". Journal of Medicinal Chemistry. 25 (8): 913–919. doi:10.1021/jm00350a006. PMID 6288945.
- ^ "Akrylfentanyl narkotikaklassas" [Acrylfentanyl is classified as a drug]. Rättsmedicinalverket [Forensic Medicine Agency] (in Swedish). 9 August 2016.
- ^ "Farligt stof i omløb - mistanken rettes mod acryl fentanyl" [Dangerous substance in circulation - suspicion is directed at Acrylfentanyl] (in Danish). mynewsdesk. 6 September 2016. Archived from the original on 9 September 2016. Retrieved 6 September 2016.
- ^ "Controlled Substances by DEA Drug Code Number" (PDF). Drug Enforcement Administration.
- ^ "31 nya ämnen kan klassas som narkotika eller hälsofarlig vara" [31 new substances can be classified as narcotic drugs or hazardous substances]. Folkhälsomyndigheten [The Public Health Authority] (in Swedish). 21 June 2016.
