Benzaldehyde
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Benzenecarbaldehyde
| |||
| Preferred IUPAC name
Benzaldehyde[1] | |||
| Other names
Benzenecarboxaldehyde
Phenylmethanal Benzoic aldehyde | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.601 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1990 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H6O | |||
| Molar mass | 106.124 g·mol−1 | ||
| Appearance | colorless liquid strongly refractive | ||
| Odor | almond-like | ||
| Density | 1.044 g/mL, liquid | ||
| Melting point | −57.12[2] °C (−70.82 °F; 216.03 K) | ||
| Boiling point | 178.1 °C (352.6 °F; 451.2 K) | ||
| 6.95 g/L (25 °C)[3] | |||
| log P | 1.64[4] | ||
| -60.78·10−6 cm3/mol | |||
Refractive index (nD)
|
1.5456 | ||
| Viscosity | 1.321 cP (25 °C) | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
−36.8 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−3525.1 kJ/mol | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H302 | |||
| P264, P270, P301+P312, P330, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 64 °C (147 °F; 337 K) | ||
| 192 °C (378 °F; 465 K) | |||
| Explosive limits | 1.4–8.5% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1300 mg/kg (rat, oral) | ||
| Safety data sheet (SDS) | J. T. Baker | ||
| Related compounds | |||
Related compounds
|
Benzyl alcohol Benzoic acid Benzaldehyde oxime | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas.[5] A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources.[6] Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods.[7]
History
[edit]Benzaldehyde was first extracted in 1803 by the French pharmacist Martrès. His experiments focused on elucidating the nature of amygdalin, the poisonous compound found in bitter almonds, the fruit of Prunus dulcis.[8] Further work on the oil by Pierre Robiquet and Antoine Boutron Charlard, two French chemists, produced benzaldehyde.[9] In 1832, Friedrich Wöhler and Justus von Liebig first synthesized benzaldehyde.[10]
Production
[edit]As of 1999, 7000 tonnes of synthetic and 100 tonnes of natural benzaldehyde were produced annually.[11] Liquid phase chlorination and oxidation of toluene are the main routes. Numerous other methods have been developed, such as the partial oxidation of benzyl alcohol, alkali hydrolysis of benzal chloride, and the carbonylation of benzene (the Gatterman-Koch reaction).[6]
A significant quantity of natural benzaldehyde is produced from cinnamaldehyde obtained from cassia oil by the retro-aldol reaction:[11] the cinnamaldehyde is heated in an aqueous/alcoholic solution between 90 °C and 150 °C with a base (most commonly sodium carbonate or bicarbonate) for 5 to 80 hours,[12] followed by distillation of the formed benzaldehyde. This reaction also yields acetaldehyde. The natural status of benzaldehyde obtained in this way is controversial.[11]
Occurrence
[edit]Benzaldehyde and similar chemicals occur naturally in many foods. Most of the benzaldehyde that people eat is from natural plant foods, such as almonds.[13]
Almonds, apricots, apples, and cherry seed contain significant amounts of amygdalin. This glycoside breaks up under enzyme catalysis into benzaldehyde, hydrogen cyanide and two equivalents of glucose.
Benzaldehyde contributes to the scent of oyster mushrooms (Pleurotus ostreatus).[14]
Reactions
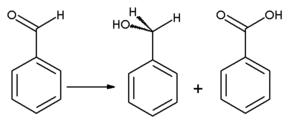
[edit]Benzaldehyde is easily oxidized to benzoic acid in air at room temperature,[15] causing a common impurity in laboratory samples. Since the boiling point of benzoic acid is much higher than that of benzaldehyde, it may be purified by distillation. Benzyl alcohol can be formed from benzaldehyde by means of hydrogenation. Reaction of benzaldehyde with anhydrous sodium acetate and acetic anhydride yields cinnamic acid, while alcoholic potassium cyanide can be used to catalyze the condensation of benzaldehyde to benzoin. Benzaldehyde undergoes disproportionation upon treatment with concentrated alkali (Cannizzaro reaction): one molecule of the aldehyde is reduced to the benzyl alcohol and another molecule is simultaneously oxidized to benzoic acid.
With diols, including many sugars, benzaldehyde condenses to form benzylidene acetals.
Uses
[edit]Benzaldehyde is commonly employed to confer almond flavor to foods and scented products, including e-cigarette liquids. It is sometimes used in cosmetics products.[16]
In industrial settings, benzaldehyde is used chiefly as a precursor to other organic compounds, ranging from pharmaceuticals to plastic additives. The aniline dye malachite green is prepared from benzaldehyde and dimethylaniline. Benzaldehyde is also a precursor to certain acridine dyes. Via aldol condensations, benzaldehyde is converted into derivatives of cinnamaldehyde and styrene. The synthesis of mandelic acid starts with the addition of hydrocyanic acid to benzaldehyde:
The resulting cyanohydrin is hydrolysed to mandelic acid. (The scheme above depicts only one of the two formed enantiomers).
Niche uses
[edit]Benzaldehyde is used as a bee repellent.[17] A small amount of benzaldehyde solution is placed on a fume board near the honeycombs. The bees then move away from the honey combs to avoid the fumes.[18] The beekeeper can then remove the honey frames from the bee hive with less risk to both bees and beekeeper.
Benzaldehyde reacts with nitroethane in the presence of a catalyst to produce phenyl-2-nitropropene, a precursor to amphetamine and other chemicals.[19]
Safety
[edit]As used in food, cosmetics, pharmaceuticals, and soap, benzaldehyde is "generally regarded as safe" (GRAS) by the US FDA[20] and FEMA.[13] This status was reaffirmed after a review in 2005.[13] It is accepted in the European Union as a flavoring agent.[16] Toxicology studies indicate that it is safe and non-carcinogenic in the concentrations used for foods and cosmetics,[16] and may even have anti-carcinogenic (anti-cancer) properties.[16]
For a 70 kg human, the lethal dose is estimated at 50 ml.[6] An acceptable daily intake of 15 mg/day has been identified for benzaldehyde by the United States Environmental Protection Agency.[21] Benzaldehyde does not accumulate in human tissues.[16] It is metabolized and then excreted in urine.[16]
References
[edit]- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 908. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Haynes, William M. (2014), CRC Handbook of Chemistry and Physics (95th ed.), CRC press, pp. 3–34, ISBN 9781482208689
- ^ "GESTIS Substance database". Institute for Occupational Safety and Health of the German Social Accident Insurance. Archived from the original on 3 March 2016. Retrieved 21 August 2012.
- ^ "Benzaldehyde_msds".
- ^ Loch, Christine; Reusch, Helmut; Ruge, Ingrid; Godelmann, Rolf; Pflaum, Tabea; Kuballa, Thomas; Schumacher, Sandra; Lachenmeier, Dirk W. (2016). "Benzaldehyde in cherry flavour as a precursor of benzene formation in beverages". Food Chemistry. 206: 74–77. doi:10.1016/j.foodchem.2016.03.034. PMID 27041300.
- ^ a b c Brühne, Friedrich and Wright, Elaine (2002) "Benzaldehyde" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_463
- ^ The Cook's Illustrated Baking Book. America's Test Kitchen. 2013. ISBN 9781936493784.
- ^ In 1803 C. Martrès published a manuscript on the oil of bitter almonds: "Recherches sur la nature et le siège de l'amertume et de l'odeur des amandes amères" (Research on the nature and location of the bitterness and the smell of bitter almonds). However, the memoir was largely ignored until an extract was published in 1819: Martrès fils (1819) "Sur les amandes amères," Journal de Pharmacie, vol. 5, pages 289–296.
- ^ Nouvelles expériences sur les amandes amères et sur l'huile volatile qu'elles fournissent Robiquet, Boutron-Charlard, Annales de chimie et de physique, 44 (1830), 352–382,
- ^ Wöhler, Friedrich and Liebig, Justus von (1832). "Untersuchungen über das Radikal der Benzoesäure" [Investigations of the radical of benzoic acid]. Annalen der Pharmacie. 3 (3): 249–282. doi:10.1002/jlac.18320030302. hdl:2027/hvd.hxdg3f.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Innovation in food engineering : new techniques and products. Passos, Maria Laura., Ribeiro, Claudio P. Boca Raton, Florida: CRC Press. 2010. p. 87. ISBN 9781420086072. OCLC 500683261.
{{cite book}}: CS1 maint: others (link) - ^ Wienes, Charles and Pittet, Alan O. (1985) U.S. patent 4,617,419 Process for preparing natural benzaldehyde and acetaldehyde, natural benzaldehyde and acetaldehyde compositions, products produced thereby and organoleptic utilities therefor.
- ^ a b c Adams, T. B.; Cohen, S. M.; Doull, J.; Feron, V. J.; Goodman, J. I.; Marnett, L. J.; Munro, I. C.; Portoghese, P. S.; Smith, R. L. (1 August 2005). "The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients". Food and Chemical Toxicology. 43 (8): 1207–1240. doi:10.1016/j.fct.2004.11.014. PMID 15950815.
- ^ Beltran-Garcia, Miguel J.; Estarron-Espinosa, Mirna; Ogura, Tetsuya (1997). "Volatile Compounds Secreted by the Oyster Mushroom (Pleurotus ostreatus) and Their Antibacterial Activities". Journal of Agricultural and Food Chemistry. 45 (10): 4049. doi:10.1021/jf960876i.
- ^ Sankar, Meenakshisundaram (2014). "The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol" (PDF). Nature Communications. 5: 3332. Bibcode:2014NatCo...5.3332S. doi:10.1038/ncomms4332. PMID 24567108.
- ^ a b c d e f Andersen, Alan (1 January 2006). "Final report on the safety assessment of benzaldehyde". International Journal of Toxicology. 25 (Suppl 1): 11–27. doi:10.1080/10915810600716612. PMID 16835129. S2CID 32177208.
- ^ Evans, Elizabeth; Butler, Carol (9 February 2010). Why Do Bees Buzz?: Why Do Bees Buzz? Fascinating Answers to Questions about Bees. Rutgers University Press. pp. 177–178. ISBN 9780813549200.
- ^ Sanford, Malcolm T.; Bonney, Richard E. (1 January 2010). Storey's Guide to Keeping Honey Bees: Honey Production, Pollination, Bee Health. Storey Publishing. p. 167. ISBN 9781603425506.
- ^ "Synthesis of Phenyl-2-Nitropropene". Erowid. Retrieved 7 October 2023.
- ^ Friedrich Brühne; Elaine Wright (2007), "Benzaldehyde", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 11
- ^ Assessment, US EPA National Center for Environmental. "Health and Environmental Effects Profile for Benzaldehyde". cfpub.epa.gov. Retrieved 16 September 2017.
External links
[edit]- International Chemical Safety Card 0102
- SIDS Initial Assessment Report for benzaldehyde from the Organisation for Economic Co-operation and Development (OECD) Archived
- Benzaldehyde description at ChemicalLand21.com
- ChemSub Online: Benzaldéhyde.
- Carcinogenicity Potency Project