Triplatin tetranitrate
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
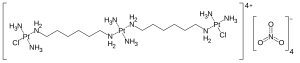
| Formula | C12H50Cl2N14O12Pt3 |
| Molar mass | 1238.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Triplatin tetranitrate (rINN; also known as BBR3464) is a platinum-based cytotoxic drug that underwent clinical trials for the treatment of human cancer.[1] The drug acts by forming adducts with cellular DNA, preventing DNA transcription and replication, thereby inducing apoptosis. Other platinum-containing anticancer drugs include cisplatin, carboplatin, and oxaliplatin.[2]
Drug development
[edit]Triplatin belongs to the anticancer class of polynuclear platinum complexes (PPCs), developed in the laboratory of Professor Nicholas Farrell, where one or more platinum centers are linked by amine ligands. BBR3464 was patented in the mid-1990s and clinical development and licensing was performed initially by Boehringer Mannheim Italia and eventually by the pharmaceutical company Roche, when clinical development was led by Novuspharma. In preclinical trials it demonstrated cytotoxic activity in cancer cell lines that had either intrinsic or acquired resistance to cisplatin. Triplatin remains the only “non-classical” platinum drug (not based on the cisplatin structure) to have entered human clinical trials. Phase I and Phase II clinical results have been summarized.[3]
Mode of action
[edit]The main target of triplatin is cellular DNA, similar to cisplatin. The drug forms novel adducts with DNA, structurally distinct from those formed by cisplatin. More recently, cellular accumulation mediated by heparan sulfate proteoglycans and high-affinity glycosaminoglycan (GAG) binding indicates that cationic PPCs are intrinsically dual-function agents, acting by mechanisms discrete from the neutral, mononuclear agents.[4]
Side effects
[edit]In phase I studies, when given once every 28 days, the main dose-limiting toxicities (DLT) of Triplatin (BBR 3464) were neutropenia and diarrhea encountered at a dose level of 1.1 mg/m2. Diarrhea was treatable with loperamide.[5] Lack of nephrotoxicity and low urinary excretion supported use of drug without hydration.[6]
References
[edit]- ^ Wheate NJ, Walker S, Craig GE, Oun R (September 2010). "The status of platinum anticancer drugs in the clinic and in clinical trials" (PDF). Dalton Transactions. 39 (35): 8113–27. doi:10.1039/C0DT00292E. hdl:2123/14271. PMID 20593091.
- ^ Farrell NP (December 2015). "Multi-platinum anti-cancer agents. Substitution-inert compounds for tumor selectivity and new targets". Chemical Society Reviews. 44 (24): 8773–85. doi:10.1039/c5cs00201j. PMID 25951946. S2CID 5171072.
- ^ Farrell NP (2012). "Progress in Platinum-Derived Drug Development". Drugs of the Future. 37 (11): 795–806. doi:10.1358/dof.2012.037.011.1830167. S2CID 75603138.
- ^ Tsotsoros SD, Lutz PB, Daniel AG, Peterson EJ, de Paiva RE, Rivera E, et al. (February 2017). "2+ for HIVNCp7 targeting". Chemical Science. 8 (2): 1269–1281. doi:10.1039/c6sc02515c. PMC 5355868. PMID 28451269.
- ^ Gourley C, Cassidy J, Edwards C, Samuel L, Bisset D, Camboni G, et al. (February 2004). "A phase I study of the trinuclear platinum compound, BBR 3464, in combination with protracted venous infusional 5-fluorouracil in patients with advanced cancer". Cancer Chemotherapy and Pharmacology. 53 (2): 95–101. doi:10.1007/s00280-003-0721-x. PMID 14605864. S2CID 19707262.
- ^ Sessa C, Capri G, Gianni L, Peccatori F, Grasselli G, Bauer J, et al. (August 2000). "Clinical and pharmacological phase I study with accelerated titration design of a daily times five schedule of BBR3464, a novel cationic triplatinum complex". Annals of Oncology. 11 (8): 977–83. doi:10.1023/a:1008302309734. PMID 11038034.
