Bisphenol A

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-(Propane-2,2-diyl)diphenol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.133 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2430 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H16O2 | |
| Molar mass | 228.291 g·mol−1 |
| Appearance | White solid |
| Odor | Phenolic, medical |
| Density | 1.217 g/cm3[1] |
| Melting point | 155 °C (311 °F; 428 K)[5] |
| Boiling point | 250–252 °C (482–486 °F; 523–525 K)[5] at 13 torrs (0.017 atm) |
| 0.3 g/L (25 °C)[2] | |
| log P | 3.41[3] |
| Vapor pressure | 5×10−6 Pa (25 °C)[4] |
| Hazards[6] | |
| GHS labelling: | |
   
| |
| Danger | |
| H317, H318, H335, H360, H411[6] | |
| P201, P202, P261, P273, P302+P352, P304+P340, P305+P351+P338, P308+P313, P333+P313, P363, P403+P233[6] | |
| NFPA 704 (fire diamond) | |
| Flash point | 227 °C (441 °F; 500 K)[6] |
| 510 °C (950 °F; 783 K)[6] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water.[2][7] BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.[8]
BPA's largest single application is as a co-monomer in the production of polycarbonates, which accounts for 65–70% of all BPA production.[9][10] The manufacturing of epoxy resins and vinyl ester resins account for 25–30% of BPA use.[9][10] The remaining 5% is used as a major component of several high-performance plastics, and as a minor additive in PVC, polyurethane, thermal paper, and several other materials. It is not a plasticizer,[11] although it is often wrongly labelled as such.
The health effects of BPA have been the subject of prolonged public and scientific debate.[12][13][14] BPA is a xenoestrogen, exhibiting hormone-like properties that mimic the effects of estrogen in the body.[15] Although the effect is very weak,[16] the pervasiveness of BPA-containing materials raises concerns, as exposure is effectively lifelong. Many BPA-containing materials are non-obvious but commonly encountered,[17] and include coatings for the inside of food cans,[18] clothing designs,[19] shop receipts,[20] and dental fillings.[21] BPA has been investigated by public health agencies in many countries, as well as by the World Health Organization.[12] While normal exposure is below the level currently associated with risk, several jurisdictions have taken steps to reduce exposure on a precautionary basis, in particular by banning BPA from baby bottles. There is some evidence that BPA exposure in infants has decreased as a result of this.[22] BPA-free plastics have also been introduced, which are manufactured using alternative bisphenols such as bisphenol S and bisphenol F, but there is also controversy around whether these are actually safer.[23][24][25]
History
[edit]Bisphenol A was first reported in 1891 by the Russian chemist Aleksandr Dianin.[26]
In 1934, workers at I.G. Farbenindustrie reported the coupling of BPA and epichlorohydrin. Over the following decade, coatings and resins derived from similar materials were described by workers at the companies of DeTrey Freres in Switzerland and DeVoe and Raynolds in the US. This early work underpinned the development of epoxy resins, which in turn motivated production of BPA.[27] The utilization of BPA further expanded with discoveries at Bayer and General Electric on polycarbonate plastics. These plastics first appeared in 1958, being produced by Mobay, General Electric, and Bayer.[28]
The British biochemist Edward Charles Dodds tested BPA as an artificial estrogen in the early 1930s.[29][30][31] Subsequent work found that it bound to estrogen receptors tens of thousands of times more weakly than estradiol, the major natural female sex hormone.[32][16] Dodds eventually developed a structurally similar compound, diethylstilbestrol (DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979.[29] BPA was never used as a drug.[29]
Production
[edit]The synthesis of BPA still follows Dianin's general method, with the fundamentals changing little in 130 years. The condensation of acetone (hence the suffix 'A' in the name)[33] with two equivalents of phenol is catalyzed by a strong acid, such as concentrated hydrochloric acid, sulfuric acid, or a solid acid resin such as the sulfonic acid form of polystyrene sulfonate.[34] An excess of phenol is used to ensure full condensation and to limit the formation of byproducts, such as Dianin's compound. BPA is fairly cheap to produce, as the synthesis benefits from a high atom economy and large amounts of both starting materials are available from the cumene process.[7] As the only by-product is water, it may be considered an industrial example of green chemistry. Global production in 2022 was estimated to be in the region of 10 million tonnes.[8]
Usually, the addition of acetone takes place at the para position on both phenols, however minor amounts of the ortho-para (up to 3%) and ortho-ortho isomers are also produced, along with several other minor by‑products.[35] These are not always removed and are known impurities in commercial samples of BPA.[36][35]
Properties
[edit]BPA has a fairly high melting point but can be easily dissolved in a broad range of organic solvents including toluene, ethanol and ethyl acetate.[37] It may be purified by recrystallisation from acetic acid with water.[38] Crystals form in the monoclinic space group P 21/n (where n indicates the glide plane); within this individual molecules of BPA are arraigned with a 91.5° torsion angle between the phenol rings.[39][40][41] Spectroscopic data is available from AIST.[42]
Uses and applications
[edit]
Main uses
[edit]Polycarbonates
[edit]About 65–70% of all bisphenol A is used to make polycarbonate plastics,[9][10] which can consist of nearly 90% BPA by mass. Polymerisation is achieved by a reaction with phosgene, conducted under biphasic conditions; the hydrochloric acid is scavenged with aqueous base.[43] This process converts the individual molecules of BPA into large polymer chains, effectively trapping them.
Epoxy and vinyl ester resins
[edit]About 25–30% of all BPA is used in the manufacture of epoxy resins and vinyl ester resins.[9][10] For epoxy resin, it is first converted to its diglycidyl ether (usually abbreviated BADGE or DGEBA).[44][45] This is achieved by a reaction with epichlorohydrin under basic conditions.
Some of this is further reacted with methacrylic acid to form bis-GMA, which is used to make vinyl ester resins. Alternatively, and to a much lesser extent, BPA may be ethoxylated and then converted to its diacrylate and dimethacrylate derivatives (bis-EMA, or EBPADMA). These may be incorporated at low levels in vinyl ester resins to change their physical properties[46] and see common use in dental composites and sealants.[47][48]
Minor uses
[edit]The remaining 5% of BPA is used in a wide range of applications, many of which involve plastic.[49] BPA is a main component of several high-performance plastics, the production of these is low compared to other plastics but still equals several thousand tons a year. Comparatively minor amounts of BPA are also used as additives or modifiers in some commodity plastics. These materials are much more common but their BPA content will be low.
Plastics
[edit]- As a major component
- Polycyanurates can be produced from BPA by way of its dicyanate ester (BADCy).[49] This is formed by a reaction between BPA and cyanogen bromide.[50] Examples include BT-Epoxy, which is one of a number of resins used in the production of printed circuit boards.
- Polyetherimides such as Ultem can be produced from BPA via a nitro-displacement of appropriate bisnitroimides.[51][52] These thermoplastic polyimide plastics have exceptional resistance to mechanical, thermal and chemical damage. They are used in medical devices and other high performance instrumentation.
- Polybenzoxazines may be produced from a number of biphenols, including BPA.[53][54]
- Polysulfones can be produced from BPA and bis(4-chlorophenyl) sulfone forming poly(bisphenol-A sulfone) (PSF). It is used as a high performance alternative to polycarbonate.[49][55]
- Bisphenol-A formaldehyde resins are a subset of phenol formaldehyde resins. They are used in the production of high-pressure laminates[49]
- As a minor component
- Polyurethane can incorporate BPA and its derivatives as hard segment chain extenders, particularly in memory foams.[56][57]
- PVC can contain BPA and its derivatives through multiple routes. BPA is sometimes used as an antioxidant in phthalates,[58] which are extensively used as plasticizers for PVC. BPA has also been used as an antioxidant to protect sensitive PVC heat stabilizers. Historically 5–10% by weight of BPA was included in barium-cadmium types, although these have largely been phased out due health concerns surrounding the cadmium. BPA diglycidyl ether (BADGE) is used as an acid scavenger, particularly in PVC dispersions, such as organosols or plastisols,[59][60] which are used as coatings for the inside of food cans, as well as embossed clothes designs produced using heat transfer vinyl or screen printing machines.[19]
- BPA is used to form a number of flame retardants used in plastics.[61] Bromination of BPA forms tetrabromobisphenol A (TBBPA), which is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistant polycarbonates by replacing some bisphenol A. A lower grade of TBBPA is used to prepare epoxy resins, used in printed circuit boards. TBBPA is also converted to tetrabromobisphenol-A-bis(2,3,-dibromopropyl ether) (TBBPA-BDBPE) which can be used as a flame retardant in polypropylene. TBBPA-BDBPE is not chemically bonded to the polymer and can leach out into the environment.[62] The use of these compounds is diminishing due to restrictions on brominated flame retardants. The reaction of BPA with phosphorus oxychloride and phenol forms bisphenol-A bis(diphenyl phosphate) (BADP), which is used as a liquid flame retarder in some high performance polymer blends such as polycarbonate/ABS mixtures.[63]
Other applications
[edit]- BPA is used as an antioxidant in several fields, particularly in brake fluids.[64]
- BPA is used as a developing agent in thermal paper (shop receipts).[20] Recycled paper products can also contain BPA,[65] although this can depend strongly on how it is recycled. Deinking can remove 95% of BPA,[9] with the pulp produced used to make newsprint, toilet paper and facial tissues. If deinking is not performed then the BPA remains in the fibers, paper recycled this way is usually made into corrugated fiberboard.[9]
- Ethoxylated BPA finds minor use as a 'levelling agent' in tin electroplating.
- Several drug candidates have also been developed from bisphenol A, including ralaniten, ralaniten acetate, and EPI-001.
BPA substitutes
[edit]Concerns about the health effects of BPA have led some manufacturers replacing it with other bisphenols, such as bisphenol S and bisphenol F. These are produced in a similar manner to BPA, by replacing acetone with other ketones, which undergo analogous condensation reactions.[7] Thus, in bisphenol F, the F signifies formaldehyde. Health concerns have also been raised about these substitutes.[66][24] Alternative polymers, such as tritan copolyester have been developed to give the same properties as polycarbonate (durable, clear) without using BPA or its analogues.
| Structural formula | Name | CAS | Reactants | |
|---|---|---|---|---|
 |
Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone |
| Bisphenol F | 620-92-8 | Phenol | Formaldehyde | |
 |
Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide |
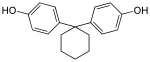 |
Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone |
 |
Tetramethyl bisphenol F | 5384-21-4 | 2,6-xylenol | Formaldehyde |
Human safety
[edit]Exposure
[edit]
As a result of the presence of BPA in plastics and other commonplace materials, most people are frequently exposed to trace levels of BPA.[67][68][69] The primary source of human exposure is via food, as epoxy and PVC are used to line the inside of food cans to prevent corrosion of the metal by acidic foodstuffs. Polycarbonate drink containers are also a source of exposure, although most disposable drinks bottles are actually made of PET, which contains no BPA. Among the non-food sources, exposure routes include through dust,[10] thermal paper,[20] clothing,[19] dental materials,[70] and medical devices.[17] Although BPA exposure is common it does not accumulate within the body, with toxicokinetic studies showing the biological half-life of BPA in adult humans to be around two hours.[71][72] The body first converts it into more water-soluble compounds via glucuronidation or sulfation, which are then removed from the body through the urine. This allows exposure to be easily determined by urine testing, facilitating convenient biomonitoring of populations.[22][17][73] Food and drink containers made from Bisphenol A-containing plastics do not contaminate the content to cause any increased cancer risk.[74]
Health effects and regulation
[edit]The health effects of BPA have been the subject of prolonged public and scientific debate,[12][13][14] with PubMed listing more than 18,000 scientific papers as of 2024.[75] Concern is mostly related to its estrogen-like activity, although it can interact with other receptor systems as an endocrine-disrupting chemical.[76] These interactions are all very weak, but exposure to BPA is effectively lifelong, leading to concern over possible cumulative effects. Studying this sort of long‑term, low‑dose interaction is difficult, and although there have been numerous studies, there are considerable discrepancies in their conclusions regarding the nature of the effects observed as well as the levels at which they occur.[12] A common criticism is that industry-sponsored trials tend to show BPA as being safer than studies performed by academic or government laboratories,[14][77] although this has also been explained in terms of industry studies being better designed.[13][78]
In the 2010s public health agencies in the EU,[79][80][81] US,[82][83] Canada,[84] Australia[85] and Japan as well as the WHO[12] all reviewed the health risks of BPA, and found normal exposure to be below the level currently associated with risk. Regardless, due to the scientific uncertainty, many jurisdictions continued to take steps to reduce exposure on a precautionary basis. In particular, infants were considered to be at greater risk,[86] leading to bans on the use of BPA in baby bottles and related products by the US,[87] Canada,[88] and EU[89] amongst others. Bottle producers largely switched from polycarbonate to polypropylene and there is some evidence that BPA exposure in infants has decreased as a result of this.[22] The European Food Safety Authority completed a re-evaluation into the risks of BPA in 2023, concluding that its tolerable daily intake should be greatly reduced.[90] This lead to a European Union resolution passed in early 2024 to ban BPA in all the food contact materials, including plastic and coated packaging. If adopted the ban would come into force after an implementation period of up to three years.
BPA exhibits very low acute toxicity (i.e. from a single large dose) as indicated by its LD50 of 4 g/kg (mouse). Reports indicate that it is a minor skin irritant as well, although less so than phenol.[7]
Pharmacology
[edit]
BPA has been found to interact with a diverse range of hormone receptors, in both humans and animals.[76] It binds to both of the nuclear estrogen receptors (ERs), ERα and ERβ. BPA is a selective estrogen receptor modulator (SERM), or partial agonist of the ER, so it can serve as both an estrogen agonist and antagonist. However, it is 1000- to 2000-fold less potent than estradiol, the major female sex hormone in humans. At high concentrations, BPA also binds to and acts as an antagonist of the androgen receptor (AR). In addition to receptor binding, the compound has been found to affect Leydig cell steroidogenesis, including affecting 17α-hydroxylase/17,20 lyase and aromatase expression and interfering with LH receptor-ligand binding.[91]
Bisphenol A's interacts with the estrogen-related receptor γ (ERR-γ). This orphan receptor (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (dissociation constant = 5.5 nM), but only weakly to the ER.[92] BPA binding to ERR-γ preserves its basal constitutive activity.[92] It can also protect it from deactivation from the SERM 4-hydroxytamoxifen (afimoxifene).[92] This may be the mechanism by which BPA acts as a xenoestrogen.[92] Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. BPA has also been found to act as an agonist of the GPER (GPR30).[93]
Environmental safety
[edit]Distribution and degradation
[edit]BPA has been detectable in the natural environment since the 1990s and is now widely distributed.[94] It is primarily a river pollutant,[95] but has also been observed in the marine environment,[96] in soils,[97] and lower levels can also be detected in air.[98] The solubility of BPA in water is low (~300 g per ton of water)[2] but this is still sufficient to make it a significant means of distribution into the environment.[97] Many of the largest sources of BPA pollution are water-based, particularly wastewater from industrial facilities using BPA. Paper recycling can be a major source of release when this includes thermal paper,[9][99] leaching from PVC items may also be a significant source,[95] as can landfill leachate.[100]
In all cases, wastewater treatment can be highly effective at removing BPA, giving reductions of 91–98%.[101] Regardless, the remaining 2–9% of BPA will continue through to the environment, with low levels of BPA commonly observed in surface water and sediment in the U.S. and Europe.[102]
Once in the environment BPA is aerobically biodegraded by a wide a variety of organisms.[94][103][104] Its half life in water has been estimated at between 4.5 and 15 days, degradation in the air is faster than this, while soil samples degrade more slowly.[97] BPA in sediment degrades most slowly of all, particularly where this is anaerobic. Abiotic degradation has been reported, but is generally slower than biodegradation. Pathways include photo-oxidation, or reactions with minerals such as goethite which may be present in soils and sediments.[105]
Environmental effects
[edit]BPA is an environmental contaminant of emerging concern.[100] Despite its short half-life and non-bioaccumulating character, the continuous release of BPA into the environment causes continuous exposure to both plant[106] and animal life. Although many studies have been performed, these often focus on a limited range of model organisms and can use BPA concentrations well beyond environmental levels.[107] As such, the precise effects of BPA on the growth, reproduction, and development of aquatic organism are not fully understood.[107] Regardless, the existing data shows the effects of BPA on wildlife to be generally negative.[108][109] BPA appears able to affect development and reproduction in a wide range of wildlife,[109][110] with certain species being particularly sensitive, such as invertebrates and amphibians.[108]
See also
[edit]- Structurally related
- 4,4'-Dihydroxybenzophenone - used as a UV stabilizer in cosmetics and plastics
- Dinitrobisphenol A - a proposed metabolite of BPA, which may show increased endocrine disrupting character
- HPTE - a metabolite of the synthetic insecticide methoxychlor
- Others
- 2,2,4,4-Tetramethyl-1,3-cyclobutanediol - next generation BPA replacement
- 4-tert-Butylphenol - used as a chain-length regulator in the production of polycarbonates and epoxy resins, it has also been studied as a potential endocrine disruptor
References
[edit]- ^ Lim CF, Tanski JM (3 August 2007). "Structural Analysis of Bisphenol-A and its Methylene, Sulfur, and Oxygen Bridged Bisphenol Analogs". Journal of Chemical Crystallography. 37 (9): 587–595. Bibcode:2007JCCry..37..587L. doi:10.1007/s10870-007-9207-8. S2CID 97284173.
- ^ a b c Shareef A, Angove MJ, Wells JD, et al. (11 May 2006). "Aqueous Solubilities of Estrone, 17β-Estradiol, 17α-Ethynylestradiol, and Bisphenol A". Journal of Chemical & Engineering Data. 51 (3): 879–881. doi:10.1021/je050318c.
- ^ Robinson BJ, Hui JP, Soo EC, et al. (2009). "Estrogenic Compounds in Seawater and Sediment from Halifax Harbour, Nova Scotia, Canada". Environmental Toxicology and Chemistry. 28 (1): 18–25. doi:10.1897/08-203.1. PMID 18702564. S2CID 13528747.
- ^ "Chemical Fact Sheet – Cas #80057 CASRN 80-05-7". speclab.com. 1 April 2012. Archived from the original on 12 February 2012. Retrieved 14 June 2012.
- ^ a b Mitrofanova SE, Bakirova IN, Zenitova LA, et al. (September 2009). "Polyurethane varnish materials based on diphenylolpropane". Russian Journal of Applied Chemistry. 82 (9): 1630–1635. doi:10.1134/S1070427209090225. S2CID 98036316.
- ^ a b c d e Sigma-Aldrich Co., Bisphenol A.
- ^ a b c d Fiege H, Voges HW, Hamamoto T, et al. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- ^ a b Abraham A, Chakraborty P (June 2020). "A review on sources and health impacts of bisphenol A". Reviews on Environmental Health. 35 (2): 201–210. doi:10.1515/reveh-2019-0034. PMID 31743105. S2CID 208186123.
- ^ a b c d e f g European Commission. Joint Research Centre. Institute for Health Consumer Protection (2010). Updated European Union risk assessment report : 4,4'-isopropylidenediphenol (bisphenol-A) : environment addendum of February 2008. Publications Office. p. 6. doi:10.2788/40195. ISBN 9789279175428.
- ^ a b c d e Vasiljevic T, Harner T (May 2021). "Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels". The Science of the Total Environment. 789: 148013. Bibcode:2021ScTEn.78948013V. doi:10.1016/j.scitotenv.2021.148013. PMID 34323825.
- ^ Cadogan DF, Howick CJ (2000). "Plasticizers". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a20_439. ISBN 3527306730.
- ^ a b c d e Joint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A : final report, including report of stakeholder meeting on bisphenol A, 1-5 November 2010, Ottawa, Canada. World Health Organization. 2011. hdl:10665/44624. ISBN 978-92-4-156427-4. Retrieved 23 March 2022.
- ^ a b c Hengstler JG, Foth H, Gebel T, et al. (April 2011). "Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A". Critical Reviews in Toxicology. 41 (4): 263–291. doi:10.3109/10408444.2011.558487. PMC 3135059. PMID 21438738.
- ^ a b c Myers JP, vom Saal FS, Akingbemi BT, et al. (March 2009). "Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A". Environmental Health Perspectives. 117 (3): 309–315. doi:10.1289/ehp.0800173. PMC 2661896. PMID 19337501.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Egan M (2013). "Sarah A. Vogel. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals". Isis. 105 (1). Berkeley: University of California Press: 254. doi:10.1086/676809. ISSN 0021-1753.
- ^ a b Blair RM (1 March 2000). "The Estrogen Receptor Relative Binding Affinities of 188 Natural and Xenochemicals: Structural Diversity of Ligands". Toxicological Sciences. 54 (1): 138–153. doi:10.1093/toxsci/54.1.138. PMID 10746941.
- ^ a b c Geens T, Aerts D, Berthot C, et al. (October 2012). "A review of dietary and non-dietary exposure to bisphenol-A" (PDF). Food and Chemical Toxicology. 50 (10): 3725–3740. doi:10.1016/j.fct.2012.07.059. PMID 22889897.
- ^ Noonan GO, Ackerman LK, Begley TH (July 2011). "Concentration of bisphenol A in highly consumed canned foods on the U.S. market". Journal of Agricultural and Food Chemistry. 59 (13): 7178–7185. doi:10.1021/jf201076f. PMID 21598963.
- ^ a b c Xue J, Liu W, Kannan K (May 2017). "Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing". Environmental Science & Technology. 51 (9): 5279–5286. Bibcode:2017EnST...51.5279X. doi:10.1021/acs.est.7b00701. PMID 28368574. Archived from the original on 29 December 2022. Retrieved 12 April 2022.
- ^ a b c Björnsdotter MK, de Boer J, Ballesteros-Gómez A (September 2017). "Bisphenol A and replacements in thermal paper: A review". Chemosphere. 182: 691–706. Bibcode:2017Chmsp.182..691B. doi:10.1016/j.chemosphere.2017.05.070. hdl:1871.1/0c9480c5-48ce-4955-8d53-39b8b246802f. PMID 28528315.
- ^ Ahovuo-Saloranta A, Forss H, Walsh T, et al. (July 2017). "Pit and fissure sealants for preventing dental decay in permanent teeth". The Cochrane Database of Systematic Reviews. 2017 (7): CD001830. doi:10.1002/14651858.CD001830.pub5. PMC 6483295. PMID 28759120.
- ^ a b c Huang RP, Liu ZH, Yin H, et al. (June 2018). "Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review". The Science of the Total Environment. 626: 971–981. Bibcode:2018ScTEn.626..971H. doi:10.1016/j.scitotenv.2018.01.144. PMID 29898562. S2CID 49194096.
- ^ Thoene M, Dzika E, Gonkowski S, et al. (February 2020). "Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review". Nutrients. 12 (2): 532. doi:10.3390/nu12020532. PMC 7071457. PMID 32092919.
- ^ a b Chen D, Kannan K, Tan H, et al. (7 June 2016). "Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review". Environmental Science & Technology. 50 (11): 5438–5453. Bibcode:2016EnST...50.5438C. doi:10.1021/acs.est.5b05387. PMID 27143250.
- ^ Eladak S, Grisin T, Moison D, et al. (2015). "A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound". Fertility and Sterility. 103 (1): 11–21. doi:10.1016/j.fertnstert.2014.11.005. PMID 25475787.
- ^ See:
- А. Дианина (1891) "О продуктахъ конденсацiи кетоновъ съ фенолами" (On condensation products of ketones with phenols), Журнал Русского физико-химического общества (Journal of the Russian Physical Chemistry Society), 23 : 488-517, 523–546, 601–611; see especially pages 491-493 ("Диметилдифенолметань" (dimethyldiphenolmethane)).
- Reprinted in condensed form in: A. Dianin (1892) "Condensationsproducte aus Ketonen und Phenolen" (Condensation products of ketones and phenols), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 25, part 3 : 334-337. doi:10.1002/cber.18920250333
- ^ Pham HQ, Marks MJ (2012). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_547.pub2. ISBN 978-3527306732.
- ^ Serini V (2000). "Polycarbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207. ISBN 978-3527306732.
- ^ a b c Vogel SA (November 2009). "The politics of plastics: the making and unmaking of bisphenol a "safety"". American Journal of Public Health. 99 (Suppl 3): S559–S566. doi:10.2105/AJPH.2008.159228. PMC 2774166. PMID 19890158.
- ^ Dodds EC, Lawson W (1936). "Synthetic Œstrogenic Agents without the Phenanthrene Nucleus". Nature. 137 (3476): 996. Bibcode:1936Natur.137..996D. doi:10.1038/137996a0. S2CID 4171635.
- ^ Dodds EC, Lawson W (1938). "Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus". Proceedings of the Royal Society of London B: Biological Sciences. 125 (839): 222–232. Bibcode:1938RSPSB.125..222D. doi:10.1098/rspb.1938.0023.
- ^ Kwon JH, Katz LE, Liljestrand HM (October 2007). "Modeling binding equilibrium in a competitive estrogen receptor binding assay". Chemosphere. 69 (7): 1025–1031. Bibcode:2007Chmsp..69.1025K. doi:10.1016/j.chemosphere.2007.04.047. PMID 17559906.
- ^ Uglea CV, Negulescu II (1991). Synthesis and Characterization of Oligomers. CRC Press. p. 103. ISBN 978-0-8493-4954-6.
- ^ De Angelis A, Ingallina P, Perego C (March 2004). "Solid Acid Catalysts for Industrial Condensations of Ketones and Aldehydes with Aromatics". Industrial & Engineering Chemistry Research. 43 (5): 1169–1178. doi:10.1021/ie030429+.
- ^ a b Terasaki M, Nomachi M, Edmonds JS, et al. (May 2004). "Impurities in industrial grade 4,4'-isopropylidene diphenol (bisphenol A): possible implications for estrogenic activity". Chemosphere. 55 (6): 927–931. Bibcode:2004Chmsp..55..927T. doi:10.1016/j.chemosphere.2003.11.063. PMID 15041297.
- ^ Pahigian JM, Zuo Y (September 2018). "Occurrence, endocrine-related bioeffects and fate of bisphenol A chemical degradation intermediates and impurities: A review". Chemosphere. 207: 469–480. Bibcode:2018Chmsp.207..469P. doi:10.1016/j.chemosphere.2018.05.117. PMID 29807346. S2CID 44172964.
- ^ Haynes WM (2017). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data (2016-2017, 97th ed.). Boca Raton, Florida: CRC Press, Inc. pp. 3–56. ISBN 9781498754293.
- ^ Perrin DD, Armarego WL (1988). Purification of laboratory chemicals. Butterworth-Heinemann. p. 208. ISBN 9780080347141.
- ^ "2,2-bis(4-Hydroxyphenyl)propane". www.ccdc.cam.ac.uk. The Cambridge Crystallographic Data Centre. Retrieved 29 June 2022.
- ^ Okada K (July 1996). "X-ray crystal structure analyses and atomic charges of color former and developer. I. Color developers". Journal of Molecular Structure. 380 (3): 223–233. Bibcode:1996JMoSt.380..223O. doi:10.1016/0022-2860(95)09168-8.
- ^ Wolak JE, Knutson J, Martin JD, et al. (1 December 2003). "Dynamic Disorder and Conformer Exchange in the Crystalline Monomer of Polycarbonate". The Journal of Physical Chemistry B. 107 (48): 13293–13299. doi:10.1021/jp036527q.
- ^ "4,4'-isopropylidenediphenol". sdbs.db.aist.go.jp. Spectral Database for Organic Compounds (SDBS). Retrieved 8 August 2024.
- ^ Serini V (2000). "Polycarbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_207. ISBN 978-3527306732.
- ^ Ng F, Couture G, Philippe C, et al. (January 2017). "Bio-Based Aromatic Epoxy Monomers for Thermoset Materials". Molecules. 22 (1): 149. doi:10.3390/molecules22010149. PMC 6155700. PMID 28106795.
- ^ Kroschwitz JI (1998). Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 5 (5 ed.). Wiley. p. 8. ISBN 978-0-471-52695-7.
- ^ Gonçalves F, Kawano Y, Pfeifer C, et al. (August 2009). "Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites". European Journal of Oral Sciences. 117 (4): 442–446. doi:10.1111/j.1600-0722.2009.00636.x. PMID 19627357.
- ^ Sideridou I, Tserki V, Papanastasiou G (April 2002). "Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins". Biomaterials. 23 (8): 1819–1829. doi:10.1016/S0142-9612(01)00308-8. PMID 11950052.
- ^ Sideridou ID, Achilias DS (July 2005). "Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC". Journal of Biomedical Materials Research Part B: Applied Biomaterials. 74B (1): 617–626. doi:10.1002/jbm.b.30252. PMID 15889433.
- ^ a b c d Geens T, Goeyens L, Covaci A (September 2011). "Are potential sources for human exposure to bisphenol-A overlooked?". International Journal of Hygiene and Environmental Health. 214 (5): 339–347. Bibcode:2011IJHEH.214..339G. doi:10.1016/j.ijheh.2011.04.005. PMID 21570349.
- ^ Hamerton I (1994). Chemistry and technology of cyanate ester resins (1st ed.). London: Blackie Academic & Professional. ISBN 978-0-7514-0044-1.
- ^ Takekoshi T, Kochanowski JE, Manello JS, et al. (June 1985). "Polyetherimides. I. Preparation of dianhydrides containing aromatic ether groups". Journal of Polymer Science: Polymer Chemistry Edition. 23 (6): 1759–1769. Bibcode:1985JPoSA..23.1759T. doi:10.1002/pol.1985.170230616.
- ^ Lau KS (2014). "10 - High-Performance Polyimides and High Temperature Resistant Polymers". Handbook of thermoset plastics (3rd ed.). San Diego: William Andrew. pp. 319–323. ISBN 978-1-4557-3107-7.
- ^ Vijayakumar CT, Shamim Rishwana S, Surender R, et al. (2 January 2014). "Structurally diverse benzoxazines: synthesis, polymerization, and thermal stability". Designed Monomers and Polymers. 17 (1): 47–57. doi:10.1080/15685551.2013.797216. S2CID 94255723.
- ^ Ghosh NN, Kiskan B, Yagci Y (November 2007). "Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties". Progress in Polymer Science. 32 (11): 1344–1391. doi:10.1016/j.progpolymsci.2007.07.002.
- ^ Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. 26 January 2001. doi:10.1002/0471238961.0118151323080920.a01. ISBN 978-0-471-48494-3.
- ^ Laza JM, Veloso A, Vilas JL (10 January 2021). "Tailoring new bisphenol a ethoxylated shape memory polyurethanes". Journal of Applied Polymer Science. 138 (2): 49660. doi:10.1002/app.49660. S2CID 224955435.
- ^ Król P (2008). Linear polyurethanes : synthesis methods, chemical structures, properties and applications. Leiden: VSP. pp. 11–14. ISBN 9789004161245.
- ^ "European Union Summary Risk Assessment Report - Bis (2-ethylhexyl) Phthalate (DEHP)". Joint Research Centre (JRC) Publications Repository. European Commission. 16 July 2008. ISSN 1018-5593. Retrieved 24 November 2021.

- ^ Shah AC, Poledna DJ (September 2003). "Review of PVC dispersion and blending resin products". Journal of Vinyl and Additive Technology. 9 (3): 146–154. doi:10.1002/vnl.10076. S2CID 98016356.
- ^ Shah AC, Poledna DJ (September 2002). "Review of specialty PVC resins". Journal of Vinyl and Additive Technology. 8 (3): 214–221. doi:10.1002/vnl.10365. S2CID 97146596.
- ^ Dagani MJ, Barda HJ, Benya TJ, et al. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405. ISBN 978-3527306732.
- ^ Gauthier LT, Laurich B, Hebert CE, et al. (20 August 2019). "Tetrabromobisphenol-A-Bis(dibromopropyl ether) Flame Retardant in Eggs, Regurgitates, and Feces of Herring Gulls from Multiple North American Great Lakes Locations". Environmental Science & Technology. 53 (16): 9564–9571. Bibcode:2019EnST...53.9564G. doi:10.1021/acs.est.9b02472. PMID 31364365. S2CID 198998658.
- ^ Pawlowski KH, Schartel B (November 2007). "Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends". Polymer International. 56 (11): 1404–1414. doi:10.1002/pi.2290.
- ^ Lamprea K, Bressy A, Mirande-Bret C, et al. (August 2018). "Alkylphenol and bisphenol A contamination of urban runoff: an evaluation of the emission potentials of various construction materials and automotive supplies" (PDF). Environmental Science and Pollution Research International. 25 (22): 21887–21900. Bibcode:2018ESPR...2521887L. doi:10.1007/s11356-018-2272-z. PMID 29796891. S2CID 44140721.
- ^ Liao C, Kannan K (November 2011). "Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure". Environmental Science & Technology. 45 (21): 9372–9379. Bibcode:2011EnST...45.9372L. doi:10.1021/es202507f. PMID 21939283.
- ^ Rochester JR, Bolden AL (July 2015). "Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes". Environmental Health Perspectives. 123 (7): 643–650. doi:10.1289/ehp.1408989. PMC 4492270. PMID 25775505.
- ^ Calafat AM, Ye X, Wong LY, et al. (January 2008). "Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004". Environmental Health Perspectives. 116 (1): 39–44. doi:10.1289/ehp.10753. PMC 2199288. PMID 18197297.
- ^ Thoene M, Rytel L, Nowicka N, et al. (May 2018). "The state of bisphenol research in the lesser developed countries of the EU: a mini-review". Toxicology Research. 7 (3): 371–380. doi:10.1039/c8tx00064f. PMC 6062254. PMID 30090587.
- ^ Vandenberg LN, Hauser R, Marcus M, et al. (August 2007). "Human exposure to bisphenol A (BPA)". Reproductive Toxicology. 24 (2): 139–177. Bibcode:2007RepTx..24..139V. doi:10.1016/j.reprotox.2007.07.010. PMID 17825522.
- ^ Van Landuyt K, Nawrot T, Geebelen B, et al. (August 2011). "How much do resin-based dental materials release? A meta-analytical approach". Dental Materials. 27 (8): 723–747. doi:10.1016/j.dental.2011.05.001. PMID 21664675.
- ^ Tsukioka T, Terasawa JI, Sato S, et al. (2004). "Development of Analytical Method for Determining Trace Amounts of BPA in Urine Samples and Estimation of Exposure to BPA". Journal of Environmental Chemistry. 14 (1): 57–63. doi:10.5985/jec.14.57.
- ^ Shin BS, Kim CH, Jun YS, et al. (December 2004). "Physiologically based pharmacokinetics of bisphenol A". Journal of Toxicology and Environmental Health. Part A. 67 (23–24): 1971–1985. Bibcode:2004JTEHA..67.1971S. doi:10.1080/15287390490514615. PMID 15513896. S2CID 24467830.
- ^ Bousoumah R, Leso V, Iavicoli I, et al. (August 2021). "Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review". Science of the Total Environment. 783: 146905. Bibcode:2021ScTEn.78346905B. doi:10.1016/j.scitotenv.2021.146905. hdl:10400.21/13242. PMID 33865140. S2CID 233290894.
- ^ "Does using plastic bottles and containers cause cancer?". Cancer Research UK. 23 December 2021.
- ^ "bisphenol a - Search Results - PubMed". PubMed. Retrieved 26 January 2024.
- ^ a b MacKay H, Abizaid A (May 2018). "A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA)". Hormones and Behavior. 101: 59–67. doi:10.1016/j.yhbeh.2017.11.001. PMID 29104009. S2CID 23088708.
- ^ vom Saal FS, Hughes C (2005). "An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment". Environ. Health Perspect. 113 (8): 926–33. doi:10.1289/ehp.7713. PMC 1280330. PMID 16079060.
- ^ Teeguarden JG, Hanson-Drury S (December 2013). "A systematic review of Bisphenol A "low dose" studies in the context of human exposure: A case for establishing standards for reporting "low-dose" effects of chemicals". Food and Chemical Toxicology. 62: 935–948. doi:10.1016/j.fct.2013.07.007. PMID 23867546.
- ^ "Bisphenol A - ECHA". echa.europa.eu. Archived from the original on 8 June 2022. Retrieved 28 March 2022.
- ^ European Food Safety Authority (2015). EFSA explains the Safety of Bisphenol A: scientific opinion on bisphenol A (2015). European Food Safety Authority. doi:10.2805/075460. ISBN 9789291996421.
- ^ "Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs". EFSA Journal. 13 (1): 3978. 21 January 2015. doi:10.2903/j.efsa.2015.3978. hdl:2164/12119.
- ^ OCSPP US EPA (21 September 2015). "Risk Management for Bisphenol A (BPA)". www.epa.gov. Retrieved 28 March 2022.
- ^ CLARITY-BPA Research Program (October 2021). NTP Research Report on the Consortium Linking Academic and Regulatory Insights on Bisphenol A Toxicity (CLARITY-BPA): A Compendium of Published Findings. p. 18. doi:10.22427/NTP-RR-18. PMID 34910417. S2CID 240266384.
- ^ Health Canada (16 April 2013). "Bisphenol A (BPA)". www.canada.ca (Health Canada). Government of Canada. Retrieved 28 March 2022.
- ^ "Bisphenol A (BPA)". Food Standards Australia New Zealand (FSANZ). Department of Health (Australia). Archived from the original on 24 March 2022. Retrieved 28 March 2022.
- ^ Aschberger K, Castello P, Hoekstra E (2010). Bisphenol A and baby bottles : challenges and perspectives. The Publications Office of the European Union. doi:10.2788/97553. ISBN 9789279158698.
- ^ "Indirect Food Additives: Polymers". Federal Register. U.S. Government Publishing Office. 17 July 2012.77 FR 41899
- ^ Legislative Services Branch (1 July 2020). "Consolidated federal laws of canada, Canada Consumer Product Safety Act". laws-lois.justice.gc.ca.
- ^ "EUR-Lex - 32011L0008 - EN - EUR-Lex". EUR-Lex. European Union.
COMMISSION DIRECTIVE 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles
- ^ Lambré C, Barat Baviera JM, Bolognesi C, et al. (April 2023). "Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs". EFSA Journal. 21 (4). doi:10.2903/j.efsa.2023.6857. PMID 37089179.
- ^ Akingbemi BT, Sottas CM, Koulova AI, et al. (1 February 2004). "Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells". Endocrinology. 145 (2): 592–603. doi:10.1210/en.2003-1174. PMID 14605012.
- ^ a b c d Matsushima A, Kakuta Y, Teramoto T, et al. (October 2007). "Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma". Journal of Biochemistry. 142 (4): 517–524. doi:10.1093/jb/mvm158. PMID 17761695.
- ^ Prossnitz ER, Barton M (May 2014). "Estrogen biology: new insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ^ a b Staples CA, Dome PB, Klecka GM, et al. (April 1998). "A review of the environmental fate, effects, and exposures of bisphenol A". Chemosphere. 36 (10): 2149–2173. Bibcode:1998Chmsp..36.2149S. doi:10.1016/S0045-6535(97)10133-3. PMID 9566294.
- ^ a b Corrales J, Kristofco LA, Steele WB, et al. (29 July 2015). "Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation". Dose-Response. 13 (3): 1559325815598308. doi:10.1177/1559325815598308. PMC 4674187. PMID 26674671.
- ^ Ozhan K, Kocaman E (February 2019). "Temporal and Spatial Distributions of Bisphenol A in Marine and Freshwaters in Turkey". Archives of Environmental Contamination and Toxicology. 76 (2): 246–254. Bibcode:2019ArECT..76..246O. doi:10.1007/s00244-018-00594-6. PMID 30610254. S2CID 58536418.
- ^ a b c Cousins I, Staples C, Kleĉka G, et al. (July 2002). "A Multimedia Assessment of the Environmental Fate of Bisphenol A". Human and Ecological Risk Assessment. 8 (5): 1107–1135. Bibcode:2002HERA....8.1107C. doi:10.1080/1080-700291905846. S2CID 43509780.
- ^ Vasiljevic T, Harner T (October 2021). "Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels". Science of the Total Environment. 789: 148013. Bibcode:2021ScTEn.78948013V. doi:10.1016/j.scitotenv.2021.148013. PMID 34323825.
- ^ Fürhacker M, Scharf S, Weber H (September 2000). "Bisphenol A: emissions from point sources". Chemosphere. 41 (5): 751–756. Bibcode:2000Chmsp..41..751F. doi:10.1016/S0045-6535(99)00466-X. PMID 10834378.
- ^ a b Qi C, Huang J, Wang B, et al. (2018). "Contaminants of emerging concern in landfill leachate in China: A review". Emerging Contaminants. 4 (1): 1–10. doi:10.1016/j.emcon.2018.06.001.
- ^ Drewes JE, Hemming J, Ladenburger SJ, et al. (2005). "An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements". Water Environment Research. 77 (1): 12–23. Bibcode:2005WaEnR..77...12D. doi:10.2175/106143005x41573. PMID 15765931. S2CID 12283834.
- ^ Klecka GM, Staples CA, Clark KE, et al. (August 2009). "Exposure analysis of bisphenol A in surface water systems in North America and Europe". Environmental Science & Technology. 43 (16): 6145–50. Bibcode:2009EnST...43.6145K. doi:10.1021/es900598e. PMID 19746705.
- ^ Kang J, Katayama Y, Kondo F (16 January 2006). "Biodegradation or metabolism of bisphenol A: From microorganisms to mammals". Toxicology. 217 (2–3): 81–90. Bibcode:2006Toxgy.217...81K. doi:10.1016/j.tox.2005.10.001. PMID 16288945.
- ^ Zhang C, Li Y, Wang C, et al. (2 January 2016). "Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review". Critical Reviews in Environmental Science and Technology. 46 (1): 1–59. Bibcode:2016CREST..46....1Z. doi:10.1080/10643389.2015.1061881. S2CID 94353391.
- ^ Im J, Löffler FE (16 August 2016). "Fate of Bisphenol A in Terrestrial and Aquatic Environments". Environmental Science & Technology. 50 (16): 8403–8416. Bibcode:2016EnST...50.8403I. doi:10.1021/acs.est.6b00877. OSTI 1470902. PMID 27401879.
- ^ Xiao C, Wang L, Zhou Q, et al. (February 2020). "Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies". Journal of Hazardous Materials. 384: 121488. Bibcode:2020JHzM..38421488X. doi:10.1016/j.jhazmat.2019.121488. PMID 31699483. S2CID 207939269.
- ^ a b Rubin AM, Seebacher F (July 2022). "Bisphenols impact hormone levels in animals: A meta-analysis". Science of the Total Environment. 828: 154533. Bibcode:2022ScTEn.82854533R. doi:10.1016/j.scitotenv.2022.154533. PMID 35288143. S2CID 247423338.
- ^ a b Wu NC, Seebacher F (July 2020). "Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis". Global Change Biology. 26 (7): 3821–3833. Bibcode:2020GCBio..26.3821W. doi:10.1111/gcb.15127. PMID 32436328. S2CID 218765595.
- ^ a b Oehlmann J, Schulte-Oehlmann U, Kloas W, et al. (2009). "A critical analysis of the biological impacts of plasticizers on wildlife". Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1526): 2047–62. doi:10.1098/rstb.2008.0242. PMC 2873012. PMID 19528055.
- ^ Wu NC, Rubin AM, Seebacher F (26 January 2022). "Endocrine disruption from plastic pollution and warming interact to increase the energetic cost of growth in a fish". Proceedings of the Royal Society B: Biological Sciences. 289 (1967). doi:10.1098/rspb.2021.2077. ISSN 0962-8452. PMC 8790379. PMID 35078359.




