Niclosamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Niclocide, Fenasal, Phenasal, others[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.052 |
| Chemical and physical data | |
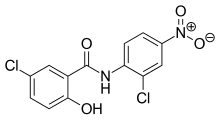
| Formula | C13H8Cl2N2O4 |
| Molar mass | 327.12 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 225 to 230 °C (437 to 446 °F) |
| |
| |
| | |
Niclosamide, sold under the brand name Niclocide among others, is an anthelmintic medication used to treat tapeworm infestations, including diphyllobothriasis, hymenolepiasis, and taeniasis. It is not effective against other worms such as flukes or roundworms.[2] It is taken by mouth.[3]
Side effects include nausea, vomiting, abdominal pain, and itchiness. It may be used during pregnancy.[3] It works by blocking glucose uptake and oxidative phosphorylation by the worm.[4]
Niclosamide was first synthesized in 1958.[5] It is on the World Health Organization's List of Essential Medicines.[6] Niclosamide is not available for human use in the United States.[7]
Side effects
[edit]Side effects include nausea, vomiting, abdominal pain, constipation, and itchiness.[3] Rarely, dizziness, skin rash, drowsiness, perianal itching, or an unpleasant taste occur. For some of these reasons, praziquantel is a preferable and equally effective treatment for tapeworm infestation.[citation needed] Important Note: Niclosamide kills the pork tapeworm and results in its digestion. This then may cause a multitude of viable eggs to be released and may result in cysticercosis. Therefore, a purge should be given 1 or two hours after treatment. CNS cysticercosis is a life-threatening condition and may require brain surgery.[8][9]
Mechanism of action
[edit]Niclosamide inhibits glucose uptake, oxidative phosphorylation, and anaerobic metabolism in the tapeworm.[10]
Other applications
[edit]Niclosamide's metabolic effects are relevant to a wide ranges of organisms, and accordingly it has been applied as a control measure to organisms other than tapeworms. For example, it is an active ingredient in some formulations such as Bayluscide for killing lamprey larvae,[11][12] as a molluscide,[13] and as a general purpose piscicide in aquaculture. Niclosamide has a short half-life in water in field conditions; this makes it valuable in ridding commercial fish ponds of unwanted fish; it loses its activity soon enough to permit re-stocking within a few days of eradicating the previous population.[13] Researchers have found that niclosamide is effective in killing invasive zebra mussels in cool waters.[14]
Research
[edit]Niclosamide is under investigation for applications against types of cancer,[15] bacterial infections,[16] or viral infections.[17][18]
In 2018, niclosamide was observed to be a potent activator of PTEN-induced kinase 1 in primary cortical neurons.[19]
References
[edit]- ^ CID 4477 from PubChem
- ^ "Niclosamide Advanced Patient Information - Drugs.com". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ a b c World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 81, 87, 591. hdl:10665/44053. ISBN 9789241547659.
- ^ Lanusse CE, Alvarez LI, Sallovitz JM, Mottier ML, Sanchez Bruni SF (13 May 2013). "Antinematodal Drugs". In Riviere JE, Papich MG (eds.). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 1096. ISBN 978-1-118-68590-7. Archived from the original on 10 September 2017.
- ^ Mehlhorn H (2008). Encyclopedia of Parasitology: A-M. Springer Science & Business Media. p. 483. ISBN 978-3-540-48994-8. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Dipylidium – Resources for Health Professionals". U.S. Centers for Disease Control and Prevention (CDC). 21 May 2020.
- ^ Remington JP, Osol A, eds. (1980). Remington's Pharmaceutical Sciences (16th ed.). Easton: Mack Pub. Co. p. 1182. OCLC 925174053.
- ^ The Merck Manual of Diagnosis and Therapy (14th ed.). Rahway: Merck Sharp & Dohme Research Laboratories. 1982. p. 176. ISBN 978-0-911910-03-2.
- ^ Weinbach EC, Garbus J (March 1969). "Mechanism of action of reagents that uncouple oxidative phosphorylation". Nature. 221 (5185): 1016–1018. Bibcode:1969Natur.221.1016W. doi:10.1038/2211016a0. PMID 4180173. S2CID 4209497.
- ^ Boogaard, Michael. "Delivery Systems of Piscicides" (PDF). Archived from the original (PDF) on 2017-06-01. Retrieved 2017-05-30.
- ^ Verdel K.Dawson (2003). "Environmental Fate and Effects of the Lampricide Bayluscide: a Review". Journal of Great Lakes Research. 29 (Supplement 1): 475–492. Bibcode:2003JGLR...29..475D. doi:10.1016/S0380-1330(03)70509-7.
- ^ a b "WHO Specifications And Evaluations. For Public Health Pesticides. Niclosamide" (PDF). Archived from the original (PDF) on 2017-01-10. Retrieved 2019-08-07.
- ^ Blank L (30 October 2018). "Researchers find new methods to combat invasive zebra mussels". The Minnesota Daily. Archived from the original on 2018-11-21. Retrieved 2018-11-19.
- ^ "Clinical Trials Using Niclosamide". NCI. Retrieved 20 March 2019.
- ^ Rajamuthiah R, Fuchs BB, Conery AL, Kim W, Jayamani E, Kwon B, et al. (April 2015). Planet PJ (ed.). "Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus". PLOS ONE. 10 (4): e0124595. Bibcode:2015PLoSO..1024595R. doi:10.1371/journal.pone.0124595. PMC 4405337. PMID 25897961.
- ^ Li Y, Li P, He Q, Zhang R, Li Y, Kamar N, et al. (January 2022). "Niclosamide inhibits hepatitis E virus through suppression of NF-kappaB signalling". Antiviral Research. 197: 105228. doi:10.1016/j.antiviral.2021.105228. PMID 34929248.
- ^ Braga L, Ali H, Secco I, Chiavacci E, Neves G, Goldhill D, et al. (June 2021). "Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia". Nature. 594 (7861): 88–93. Bibcode:2021Natur.594...88B. doi:10.1038/s41586-021-03491-6. PMC 7611055. PMID 33827113.
- ^ Barini E, Miccoli A, Tinarelli F, Mulholland K, Kadri H, Khanim F, et al. (March 2018). "The Anthelmintic Drug Niclosamide and Its Analogues Activate the Parkinson's Disease Associated Protein Kinase PINK1". ChemBioChem. 19 (5): 425–429. doi:10.1002/cbic.201700500. PMC 5901409. PMID 29226533.
Further reading
[edit]- Taber CW, Venes D, Thomas CL (2001). Taber's Cyclopedic Medical Cictionary (19th ed.). Philadelphia: F.A.Davis Co. ISBN 978-0-8036-0655-5.
- World Health Organization (1995). "Helminths: Cestode (tapeworm) infection: Niclosamide". WHO model prescribing information : drugs used in parasitic diseases (2nd ed.). World Health Organization (WHO). hdl:10665/41765. ISBN 9789241401043.
External links
[edit]- "Niclosamide". Drug Information Portal. U.S. National Library of Medicine.
- Niclosamide in the Pesticide Properties DataBase (PPDB)
- "MedlinePlus Drug Information: Niclosamide (Oral)". MedlinePlus. U.S. National Library of Medicine. 1995-06-23. Archived from the original on 2006-12-16.
