Fipexide
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.128 |
| Chemical and physical data | |
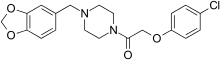
| Formula | C20H21ClN2O4 |
| Molar mass | 388.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fipexide (Attentil, Vigilor) is a psychoactive drug of the piperazine chemical class which was developed in Italy in 1983.[1] It was used as a nootropic drug in Italy and France, mainly for the treatment of senile dementia,[2] but is no longer in common use due to the occurrence of rare adverse drug reactions including fever[3] and hepatitis. Fipexide is similar in action to other nootropic drugs such as piracetam[citation needed] and has a few similarities in chemical structure to centrophenoxine. Chemically, it is an amide union of parachlorophenoxyacetate and methylenedioxybenzylpiperazine (MDBZP), and has been shown to metabolize to the latter, which plays a significant role in its effects[citation needed].
Synthesis
[edit]
PTC alkylation of piperazine (1) with 2 equivalents of piperonyl chloride [25054-53-9] (2) in the presence of cetrimonium bromide gives 1,4-bis-piperonylpiperazine [55436-41-4] (3). Base catalyzed treatment with 4-Chlorophenoxyacetic acid (4) displaces one of the piperonyl groups to give fipexide (5).
See also
[edit]References
[edit]- ^ Missale C, Pasinetti G, Govoni S, Spano PF, Trabucchi M (February 1983). "[Fipexide: a new drug for the regulation of dopaminergic system at the macromolecular level]". Bollettino Chimico Farmaceutico (in Italian). 122 (2): 79–85. PMID 6871040.
- ^ Bompani R, Scali G (1986). "Fipexide, an effective cognition activator in the elderly: a placebo-controlled, double-blind clinical trial". Current Medical Research and Opinion. 10 (2): 99–106. doi:10.1185/03007998609110426. PMID 3519097.
- ^ Guy C, Blay N, Rousset H, Fardeau V, Ollagnier M (1990). "[Fever caused by fipexide. Evaluation of the national pharmacovigilance survey]". Therapie (in French). 45 (5): 429–31. PMID 2260037.
- ^ Gardini, G. P.; Palla, G.; Scapini, G.; Cesaroni, M. R. (2006). "Convenient Synthesis of N-Benzyl-N′-acyl-piperazines". Synthetic Communications. 12 (11): 887–890. ISSN 0039-7911. doi:10.1080/00397918208065967.
- ^ Anon., FR 7524M (1969-12-15).
- ^ Gian P. Gardini, Giancarlo Scapini, Armando Raimondi, Placido Poidomani, U.S. patent 4,225,714 (1980 to Farmaceutici Geymonat Sud S.P.A.).
