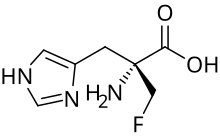
α-Fluoromethylhistidine
| |
| Names | |
|---|---|
| Other names
α-FMH
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H10FN3O2 | |
| Molar mass | 187.174 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
α-Fluoromethylhistidine (α-FMH) is an irreversible specific inhibitor of histidine decarboxylase (HDC).[1] It functions by forming a covalent linkage with a catalytic serine residue on the active site of HDC. Due to its efficacy in reducing histamine levels in tissue mast cells, it has many applications in the study of histaminergic systems.[2]
It has potent sleep-inducing effects in mice.[3][4][5] In the central nervous systems of rats, α-FMH administration has been shown to cause impairments in long-term memory and learning.[6] Additionally, injection of α-FMH has been shown to increase food intake, although the mechanism is believed to distinct from HDC inhibition, indicating that α-FMH may be involved in the regulation of non-histaminergic systems. A proposed mechanism involves the enhanced expression of neuropeptide Y (NPY) rather than HDC inhibition.[7]
α-FMH has also been shown to target isozymes of the glutathione S-transferase (GST) family. Due to the role of GSTs in detoxification, the efficacy of HDC inhibition by α-FMH in humans and its potential for the treatment of pathological conditions is subject to further research.[8]
See also
[edit]References
[edit]- ^ August TF, Musson DG, Hwang SS, Duggan DE, Hooke KF, Roman IJ, et al. (August 1985). "Bioanalysis and disposition of alpha-fluoromethylhistidine, a new histidine decarboxylase inhibitor". Journal of Pharmaceutical Sciences. 74 (8): 871–5. doi:10.1002/jps.2600740814. PMID 4032273.
- ^ Watanabe T, Yamatodani A, Maeyama K, Wada H (September 1990). "Pharmacology of alpha-fluoromethylhistidine, a specific inhibitor of histidine decarboxylase". Trends in Pharmacological Sciences. 11 (9): 363–7. doi:10.1016/0165-6147(90)90181-7. PMID 2238092.
- ^ Thakkar MM (February 2011). "Histamine in the regulation of wakefulness". Sleep Medicine Reviews. 15 (1): 65–74. doi:10.1016/j.smrv.2010.06.004. PMC 3016451. PMID 20851648.
- ^ Monti JM, D'Angelo L, Jantos H, Pazos S (1988). "Effects of a-fluoromethylhistidine on sleep and wakefulness in the rat. Short note". Journal of Neural Transmission. 72 (2): 141–5. doi:10.1007/bf01250237. PMID 3385425. S2CID 20689507.
- ^ Oishi R, Itoh Y, Nishibori M, Saeki K, Ueki S (1988). "Enhancement by alpha-fluoromethylhistidine of the thiopental sleep-prolonging action of delta 9-tetrahydrocannabinol". Psychopharmacology. 95 (1): 77–81. doi:10.1007/bf00212771. PMID 2838862. S2CID 43979118.
- ^ Benetti F, Furini CR, de Carvalho Myskiw J, Provensi G, Passani MB, Baldi E, et al. (May 2015). "Histamine in the basolateral amygdala promotes inhibitory avoidance learning independently of hippocampus". Proceedings of the National Academy of Sciences of the United States of America. 112 (19): E2536-42. Bibcode:2015PNAS..112E2536B. doi:10.1073/pnas.1506109112. PMC 4434720. PMID 25918368.
- ^ Ishizuka T, Isono H, Ohtsu H, Yamatodani A (March 2004). "A possible mechanism of alpha-fluoromethylhistidine-induced increase of food intake". Inflammation Research. 53 (Suppl 1): S45-6. doi:10.1007/s00011-003-0321-6. PMID 15054612. S2CID 6200822.
- ^ Considine KL, Stefanidis L, Grozinger KG, Audie J, Alper BJ (March 2017). "Efficient synthesis of α-fluoromethylhistidine di-hydrochloride and demonstration of its efficacy as a glutathione S-transferase inhibitor". Bioorganic & Medicinal Chemistry Letters. 27 (6): 1335–1340. doi:10.1016/j.bmcl.2017.02.024. PMID 28228363.
