DNA vaccine

A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.[1][2]
DNA vaccines work by injecting genetically engineered plasmid containing the DNA sequence encoding the antigen(s) against which an immune response is sought, so the cells directly produce the antigen, thus causing a protective immunological response.[3] DNA vaccines have theoretical advantages over conventional vaccines, including the "ability to induce a wider range of types of immune response".[4] Several DNA vaccines have been tested for veterinary use.[3] In some cases, protection from disease in animals has been obtained, in others not.[3] Research is ongoing over the approach for viral, bacterial and parasitic diseases in humans, as well as for cancers.[4] In August 2021, Indian authorities gave emergency approval to ZyCoV-D. Developed by Cadila Healthcare, it is the first DNA vaccine approved for humans.[5]
History
[edit]Conventional vaccines contain either specific antigens from a pathogen, or attenuated viruses which stimulate an immune response in the vaccinated organism. DNA vaccines are members of the genetic vaccines, because they contain a genetic information (DNA or RNA) that codes for the cellular production (protein biosynthesis) of an antigen. DNA vaccines contain DNA that codes for specific antigens from a pathogen. The DNA is injected into the body and taken up by cells, whose normal metabolic processes synthesize proteins based on the genetic code in the plasmid that they have taken up. Because these proteins contain regions of amino acid sequences that are characteristic of bacteria or viruses, they are recognized as foreign and when they are processed by the host cells and displayed on their surface, the immune system is alerted, which then triggers immune responses.[6][7] Alternatively, the DNA may be encapsulated in protein to facilitate cell entry. If this capsid protein is included in the DNA, the resulting vaccine can combine the potency of a live vaccine without reversion risks.[citation needed]
In 1983, Enzo Paoletti and Dennis Panicali at the New York Department of Health devised a strategy to produce recombinant DNA vaccines by using genetic engineering to transform ordinary smallpox vaccine into vaccines that may be able to prevent other diseases.[8] They altered the DNA of cowpox virus by inserting a gene from other viruses (namely Herpes simplex virus, hepatitis B and influenza).[9][10] In 1993, Jeffrey Ulmer and co-workers at Merck Research Laboratories demonstrated that direct injection of mice with plasmid DNA encoding a flu antigen protected the animals against subsequent experimental infection with influenza virus.[11] In 2016 a DNA vaccine for the Zika virus began testing in humans at the National Institutes of Health. The study was planned to involve up to 120 subjects aged between 18 and 35. Separately, Inovio Pharmaceuticals and GeneOne Life Science began tests of a different DNA vaccine against Zika in Miami. The NIH vaccine is injected into the upper arm under high pressure. Manufacturing the vaccines in volume remained unsolved as of August 2016.[12] Clinical trials for DNA vaccines to prevent HIV are underway.[13]
In August 2021, Indian authorities gave emergency approval to ZyCoV-D. Developed by Cadila Healthcare, it is the first DNA vaccine against COVID-19.[5]
Applications
[edit]As of 2021[update] no DNA vaccines have been approved for human use in the United States. Few experimental trials have evoked a response strong enough to protect against disease and the technique's usefulness remains to be proven in humans.
A veterinary DNA vaccine to protect horses from West Nile virus has been approved.[14] Another West Nile virus vaccine has been tested successfully on American robins.[15]
DNA immunization is also being investigated as a means of developing antivenom sera.[1] DNA immunization can be used as a technology platform for monoclonal antibody induction.[2]
Advantages
[edit]- No risk for infections[7]
- Antigen presentation by both MHC class I and class II molecules[7]
- Polarise T-cell response toward type 1 or type 2[7]
- Immune response focused on the antigen of interest
- Ease of development and production[7]
- Stability for storage and shipping
- Cost-effectiveness
- Obviates need for peptide synthesis, expression and purification of recombinant proteins and use of toxic adjuvants[16]
- Long-term persistence of immunogen[6]
- In vivo expression ensures protein more closely resembles normal eukaryotic structure, with accompanying post-translational modifications[6]
Disadvantages
[edit]- Limited to protein immunogens (not useful for non-protein based antigens such as bacterial polysaccharides)
- Potential for atypical processing of bacterial and parasite proteins[7]
- Potential when using nasal spray administration of plasmid DNA nanoparticles to transfect non-target cells, such as brain cells[17]
- Cross-contamination when manufacturing different types of live vaccines in same facility
Plasmid vectors
[edit]Vector design
[edit]DNA vaccines elicit the best immune response when high-expression vectors are used. These are plasmids that usually consist of a strong viral promoter to drive the in vivo transcription and translation of the gene (or complementary DNA) of interest.[18] Intron A may sometimes be included to improve mRNA stability and hence increase protein expression.[19] Plasmids also include a strong polyadenylation/transcriptional termination signal, such as bovine growth hormone or rabbit beta-globulin polyadenylation sequences.[6][7][20] Polycistronic vectors (with multiple genes of interest) are sometimes constructed to express more than one immunogen, or to express an immunogen and an immunostimulatory protein.[21]
Because the plasmid – carrying relatively small genetic code up to about 200 Kbp – is the "vehicle" from which the immunogen is expressed, optimising vector design for maximal protein expression is essential.[21] One way of enhancing protein expression is by optimising the codon usage of pathogenic mRNAs for eukaryotic cells. Pathogens often have different AT-contents than the target species, so altering the gene sequence of the immunogen to reflect the codons more commonly used in the target species may improve its expression.[22]
Another consideration is the choice of promoter. The SV40 promoter was conventionally used until research showed that vectors driven by the Rous Sarcoma Virus (RSV) promoter had much higher expression rates.[6] More recently, expression and immunogenicity have been further increased in model systems by the use of the cytomegalovirus (CMV) immediate early promoter, and a retroviral cis-acting transcriptional element.[23] Additional modifications to improve expression rates include the insertion of enhancer sequences, synthetic introns, adenovirus tripartite leader (TPL) sequences and modifications to the polyadenylation and transcriptional termination sequences.[6] An example of DNA vaccine plasmid is pVAC, which uses SV40 promoter.
Structural instability phenomena are of particular concern for plasmid manufacture, DNA vaccination and gene therapy.[24] Accessory regions pertaining to the plasmid backbone may engage in a wide range of structural instability phenomena. Well-known catalysts of genetic instability include direct, inverted and tandem repeats, which are conspicuous in many commercially available cloning and expression vectors. Therefore, the reduction or complete elimination of extraneous noncoding backbone sequences would pointedly reduce the propensity for such events to take place and consequently the overall plasmid's recombinogenic potential.[25]
Mechanism of plasmids
[edit]Once the plasmid inserts itself into the transfected cell nucleus, it codes for a peptide string of a foreign antigen. On its surface the cell displays the foreign antigen with both histocompatibility complex (MHC) classes I and class II molecules. The antigen-presenting cell then travels to the lymph nodes and presents the antigen peptide and costimulatory molecule signalling to T-cell, initiating the immune response.[26]
Vaccine insert design
[edit]Immunogens can be targeted to various cellular compartments to improve antibody or cytotoxic T-cell responses. Secreted or plasma membrane-bound antigens are more effective at inducing antibody responses than cytosolic antigens, while cytotoxic T-cell responses can be improved by targeting antigens for cytoplasmic degradation and subsequent entry into the major histocompatibility complex (MHC) class I pathway.[7] This is usually accomplished by the addition of N-terminal ubiquitin signals.[27][28][29]
The conformation of the protein can also affect antibody responses. "Ordered" structures (such as viral particles) are more effective than unordered structures.[30] Strings of minigenes (or MHC class I epitopes) from different pathogens raise cytotoxic T-cell responses to some pathogens, especially if a TH epitope is also included.[7]
Delivery
[edit]
DNA vaccines have been introduced into animal tissues by multiple methods. In 1999, the two most popular approaches were injection of DNA in saline: by using a standard hypodermic needle, or by using a gene gun delivery.[31] Several other techniques have been documented in the intervening years.
Saline injection
[edit]Injection in saline is normally conducted intramuscularly (IM) in skeletal muscle, or intradermally (ID), delivering DNA to extracellular spaces. This can be assisted either 1) by electroporation;[32] 2) by temporarily damaging muscle fibres with myotoxins such as bupivacaine; or 3) by using hypertonic solutions of saline or sucrose.[6] Immune responses to this method can be affected by factors including needle type,[16] needle alignment, speed of injection, volume of injection, muscle type, and age, sex and physiological condition of the recipient.[6]
Gene gun
[edit]Gene gun delivery ballistically accelerates plasmid DNA (pDNA) that has been absorbed onto gold or tungsten microparticles into the target cells, using compressed helium as an accelerant.[6][21]
Mucosal surface delivery
[edit]Alternatives included aerosol instillation of naked DNA on mucosal surfaces, such as the nasal and lung mucosa,[21] and topical administration of pDNA to the eye[33] and vaginal mucosa.[21] Mucosal surface delivery has also been achieved using cationic liposome-DNA preparations,[7] biodegradable microspheres,[34][21] attenuated Salmonalla,[35] Shigella or Listeria vectors for oral administration to the intestinal mucosa[36] and recombinant adenovirus vectors.[21]
Polymer vehicle
[edit]A hybrid vehicle composed of bacteria cell and synthetic polymers has been employed for DNA vaccine delivery. An E. coli inner core and poly(beta-amino ester) outer coat function synergistically to increase efficiency by addressing barriers associated with antigen-presenting cell gene delivery which include cellular uptake and internalization, phagosomal escape and intracellular cargo concentration.[jargon] Tested in mice, the hybrid vector was found to induce immune response.[37][38]
ELI immunization
[edit]Another approach to DNA vaccination is expression library immunization (ELI). Using this technique, potentially all the genes from a pathogen can be delivered at one time, which may be useful for pathogens that are difficult to attenuate or culture.[6] ELI can be used to identify which genes induce a protective response. This has been tested with Mycoplasma pulmonis, a murine lung pathogen with a relatively small genome. Even partial expression libraries can induce protection from subsequent challenge.[39]
Helpful tabular comparison
[edit]| Method of delivery | Formulation of DNA | Target tissue | Amount of DNA | |
|---|---|---|---|---|
| Parenteral | Injection (hypodermic needle) | Aqueous solution in saline | IM (skeletal); ID; (IV, subcutaneous and intraperitoneal with variable success) | Large amounts (approximately 100-200 μg) |
| Gene gun | DNA-coated gold beads | ED (abdominal skin); vaginal mucosa; surgically exposed muscle and other organs | Small amounts (as little as 16 ng) | |
| Pneumatic (jet) injection | Aqueous solution | ED | Very high (as much as 300 μg) | |
| Topical application | Aqueous solution | Ocular; intravaginal | Small amounts (up to 100 μg) | |
| Cytofectin-mediated[jargon] | Liposomes (cationic); microspheres; recombinant adenovirus vectors; attenuated Shigella vector; aerosolised cationic lipid formulations | IM; IV (to transfect tissues systemically); intraperitoneal; oral immunization to the intestinal mucosa; nasal/lung mucosal membranes | variable | |
| Method of delivery | Advantage | Disadvantage |
|---|---|---|
| Intramuscular or Intradermal injection |
|
|
| Gene gun |
|
|
| Jet injection |
|
|
| Liposome-mediated delivery |
|
|
Dosage
[edit]The delivery method determines the dose required to raise an effective immune response. Saline injections require variable amounts of DNA, from 10 μg to 1 mg, whereas gene gun deliveries require 100 to 1000 times less.[40] Generally, 0.2 μg – 20 μg are required, although quantities as low as 16 ng have been reported.[6] These quantities vary by species. Mice for example, require approximately 10 times less DNA than primates.[7] Saline injections require more DNA because the DNA is delivered to the extracellular spaces of the target tissue (normally muscle), where it has to overcome physical barriers (such as the basal lamina and large amounts of connective tissue) before it is taken up by the cells, while gene gun deliveries drive/force DNA directly into the cells, resulting in less "wastage".[6][7]
Immune response
[edit]Helper T cell responses
[edit]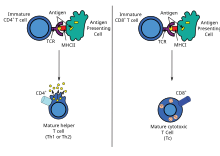
DNA immunization can raise multiple TH responses, including lymphoproliferation and the generation of a variety of cytokine profiles. A major advantage of DNA vaccines is the ease with which they can be manipulated to bias the type of T-cell help towards a TH1 or TH2 response.[41] Each type has distinctive patterns of lymphokine and chemokine expression, specific types of immunoglobulins, patterns of lymphocyte trafficking and types of innate immune responses.
Other types of T-cell help
[edit]The type of T-cell help raised is influenced by the delivery method and the type of immunogen expressed, as well as the targeting of different lymphoid compartments.[6][42] Generally, saline needle injections (either IM or ID) tend to induce TH1 responses, while gene gun delivery raises TH2 responses.[41][42] This is true for intracellular and plasma membrane-bound antigens, but not for secreted antigens, which seem to generate TH2 responses, regardless of the method of delivery.[43]
Generally the type of T-cell help raised is stable over time, and does not change when challenged or after subsequent immunizations that would normally have raised the opposite type of response in a naïve specimen.[41][42] However, Mor et al.. (1995)[18] immunized and boosted mice with pDNA encoding the circumsporozoite protein of the mouse malarial parasite Plasmodium yoelii (PyCSP) and found that the initial TH2 response changed, after boosting, to a TH1 response.
Basis for different types of T-cell help
[edit]How these different methods operate, the forms of antigen expressed, and the different profiles of T-cell help is not understood. It was thought that the relatively large amounts of DNA used in IM injection were responsible for the induction of TH1 responses. However, evidence shows no dose-related differences in TH type.[41] The type of T-cell help raised is determined by the differentiated state of antigen presenting cells. Dendritic cells can differentiate to secrete IL-12 (which supports TH1 cell development) or IL-4 (which supports TH2 responses).[44] pDNA injected by needle is endocytosed into the dendritic cell, which is then stimulated to differentiate for TH1 cytokine (IL-12) production,[45] while the gene gun bombards the DNA directly into the cell, thus bypassing TH1 stimulation.
Practical uses of polarised T-cell help
[edit]Polarisation in T-cell help is useful in influencing allergic responses and autoimmune diseases. In autoimmune diseases, the goal is to shift the self-destructive TH1 response (with its associated cytotoxic T cell activity) to a non-destructive TH2 response. This has been successfully applied in predisease priming for the desired type of response in preclinical models[7] and is somewhat successful in shifting the response for an established disease.[46]
Cytotoxic T-cell responses
[edit]One of the advantages of DNA vaccines is that they are able to induce cytotoxic T lymphocytes (CTL) without the inherent risk associated with live vaccines. CTL responses can be raised against immunodominant and immunorecessive CTL epitopes,[47] as well as subdominant CTL epitopes,[34][jargon] in a manner that appears to mimic natural infection. This may prove to be a useful tool in assessing CTL epitopes and their role in providing immunity.
Cytotoxic T-cells recognise small peptides (8-10 amino acids) complexed to MHC class I molecules.[48] These peptides are derived from cytosolic proteins that are degraded and delivered to the nascent MHC class I molecule within the endoplasmic reticulum (ER).[48] Targeting gene products directly to the ER (by the addition of an ER insertion signal sequence at the N-terminus) should thus enhance CTL responses. This was successfully demonstrated using recombinant vaccinia viruses expressing influenza proteins,[48] but the principle should also be applicable to DNA vaccines. Targeting antigens for intracellular degradation (and thus entry into the MHC class I pathway) by the addition of ubiquitin signal sequences, or mutation of other signal sequences, was shown to be effective at increasing CTL responses.[28]
CTL responses can be enhanced by co-inoculation with co-stimulatory molecules such as B7-1 or B7-2 for DNA vaccines against influenza nucleoprotein,[47][49] or GM-CSF for DNA vaccines against the murine malaria model P. yoelii.[50] Co-inoculation with plasmids encoding co-stimulatory molecules IL-12 and TCA3 were shown to increase CTL activity against HIV-1 and influenza nucleoprotein antigens.[49][51]
Humoral (antibody) response
[edit]
Antibody responses elicited by DNA vaccinations are influenced by multiple variables, including antigen type; antigen location (i.e. intracellular vs. secreted); number, frequency and immunization dose; site and method of antigen delivery.
Kinetics of antibody response
[edit]Humoral responses after a single DNA injection can be much longer-lived than after a single injection with a recombinant protein. Antibody responses against hepatitis B virus (HBV) envelope protein (HBsAg) have been sustained for up to 74 weeks without boost, while lifelong maintenance of protective response to influenza haemagglutinin was demonstrated in mice after gene gun delivery.[52] Antibody-secreting cells (ASC) migrate to the bone marrow and spleen for long-term antibody production, and generally localise there after one year.[52]
Comparisons of antibody responses generated by natural (viral) infection, immunization with recombinant protein and immunization with pDNA are summarised in Table 4. DNA-raised antibody responses rise much more slowly than when natural infection or recombinant protein immunization occurs. As many as 12 weeks may be required to reach peak titres in mice, although boosting can decrease the interval. This response is probably due to the low levels of antigen expressed over several weeks, which supports both primary and secondary phases of antibody response.[clarification needed] DNA vaccine expressing HBV small and middle envelope protein was injected into adults with chronic hepatitis. The vaccine resulted in specific interferon gamma cell production. Also specific T-cells for middle envelop proteins antigens were developed. The immune response of the patients was not robust enough to control HBV infection[53]
| Method of Immunization | |||
|---|---|---|---|
| DNA vaccine | Recombinant protein | Natural infection | |
| Amount of inducing antigen | ng | μg | ? (ng-μg) |
| Duration of antigen presentation | several weeks | < 1 week | several weeks |
| Kinetics of antibody response | slow rise | rapid rise | rapid rise |
| Number of inoculations to obtain high avidity IgG and migration of ASC to bone marrow | one | two | one |
| Ab isotype (murine models) | C’-dependent or C’-independent | C’-dependent | C’-independent |
Additionally, the titres of specific antibodies raised by DNA vaccination are lower than those obtained after vaccination with a recombinant protein. However, DNA immunization-induced antibodies show greater affinity to native epitopes than recombinant protein-induced antibodies. In other words, DNA immunization induces a qualitatively superior response. Antibodies can be induced after one vaccination with DNA, whereas recombinant protein vaccinations generally require a boost. DNA immunization can be used to bias the TH profile of the immune response and thus the antibody isotype, which is not possible with either natural infection or recombinant protein immunization. Antibody responses generated by DNA are useful as a preparative tool. For example, polyclonal and monoclonal antibodies can be generated for use as reagents.[citation needed]
Mechanistic basis for DNA-raised immune responses
[edit]DNA uptake mechanism
[edit]When DNA uptake and subsequent expression was first demonstrated in vivo in muscle cells,[54] these cells were thought to be unique because of their extensive network of T-tubules. Using electron microscopy, it was proposed that DNA uptake was facilitated by caveolae (or, non-clathrin coated pits).[55] However, subsequent research revealed that other cells (such as keratinocytes, fibroblasts and epithelial Langerhans cells) could also internalize DNA.[46][56] The mechanism of DNA uptake is not known.
Two theories dominate – that in vivo uptake of DNA occurs non-specifically, in a method similar to phago- or pinocytosis,[21] or through specific receptors.[57] These might include a 30kDa surface receptor, or macrophage scavenger receptors.[clarification needed] The 30kDa surface receptor binds specifically to 4500-bp DNA fragments (which are then internalised) and is found on professional APCs and T-cells. Macrophage scavenger receptors bind to a variety of macromolecules, including polyribonucleotides and are thus candidates for DNA uptake.[57][58] Receptor-mediated DNA uptake could be facilitated by the presence of polyguanylate sequences.[clarification needed][citation needed] Gene gun delivery systems, cationic liposome packaging, and other delivery methods bypass this entry method, but understanding it may be useful in reducing costs (e.g. by reducing the requirement for cytofectins), which could be important in animal husbandry.
Antigen presentation by bone marrow-derived cells
[edit]
Studies using chimeric mice have shown that antigen is presented by bone-marrow derived cells, which include dendritic cells, macrophages and specialised B-cells called professional antigen presenting cells (APC).[49][59] After gene gun inoculation to the skin, transfected Langerhans cells migrate to the draining lymph node to present antigens.[7] After IM and ID injections, dendritic cells present antigen in the draining lymph node[56] and transfected macrophages have been found in the peripheral blood.[60]
Besides direct transfection of dendritic cells or macrophages, cross priming occurs following IM, ID and gene gun DNA deliveries. Cross-priming occurs when a bone marrow-derived cell presents peptides from proteins synthesised in another cell in the context of MHC class 1. This can prime cytotoxic T-cell responses and seems to be important for a full primary immune response.[7][61]
Target site role
[edit]IM and ID DNA delivery initiate immune responses differently. In the skin, keratinocytes, fibroblasts and Langerhans cells take up and express antigens and are responsible for inducing a primary antibody response. Transfected Langerhans cells migrate out of the skin (within 12 hours) to the draining lymph node where they prime secondary B- and T-cell responses. In skeletal muscle, striated muscle cells are most frequently transfected, but seem to be unimportant in immune response. Instead, IM inoculated DNA "washes" into the draining lymph node within minutes, where distal dendritic cells are transfected and then initiate an immune response. Transfected myocytes seem to act as a "reservoir" of antigen for trafficking professional APCs.[21][54][61]
Maintenance of immune response
[edit]DNA vaccination generates an effective immune memory via the display of antigen-antibody complexes on follicular dendritic cells (FDC), which are potent B-cell stimulators. T-cells can be stimulated by similar, germinal centre dendritic cells. FDC are able to generate an immune memory because antibodies production "overlaps" long-term expression of antigen, allowing antigen-antibody immunocomplexes to form and be displayed by FDC.[7]
Interferons
[edit]Both helper and cytotoxic T-cells can control viral infections by secreting interferons. Cytotoxic T cells usually kill virally infected cells. However, they can also be stimulated to secrete antiviral cytokines such as IFN-γ and TNF-α, which do not kill the cell, but limit viral infection by down-regulating the expression of viral components.[62] DNA vaccinations can be used to curb viral infections by non-destructive IFN-mediated control. This was demonstrated for hepatitis B.[63] IFN-γ is critically important in controlling malaria infections[64] and is a consideration for anti-malarial DNA vaccines.
Immune response modulation
[edit]Cytokine modulation
[edit]An effective vaccine must induce an appropriate immune response for a given pathogen. DNA vaccines can polarise T-cell help towards TH1 or TH2 profiles and generate CTL and/or antibody when required. This can be accomplished by modifications to the form of antigen expressed (i.e. intracellular vs. secreted), the method and route of delivery or the dose.[41][42][65][66][67] It can also be accomplished by the co-administration of plasmid DNA encoding immune regulatory molecules, i.e. cytokines, lymphokines or co-stimulatory molecules. These "genetic adjuvants" can be administered as a:
- mixture of 2 plasmids, one encoding the immunogen and the other encoding the cytokine
- single bi- or polycistronic vector, separated by spacer regions
- plasmid-encoded chimera, or fusion protein
In general, co-administration of pro-inflammatory agents (such as various interleukins, tumor necrosis factor, and GM-CSF) plus TH2-inducing cytokines increase antibody responses, whereas pro-inflammatory agents and TH1-inducing cytokines decrease humoral responses and increase cytotoxic responses (more important in viral protection). Co-stimulatory molecules such as B7-1, B7-2 and CD40L are sometimes used.
This concept was applied in topical administration of pDNA encoding IL-10.[33] Plasmid encoding B7-1 (a ligand on APCs) successfully enhanced the immune response in tumour models. Mixing plasmids encoding GM-CSF and the circumsporozoite protein of P. yoelii (PyCSP) enhanced protection against subsequent challenge (whereas plasmid-encoded PyCSP alone did not). It was proposed that GM-CSF caused dendritic cells to present antigen more efficiently and enhance IL-2 production and TH cell activation, thus driving the increased immune response.[50] This can be further enhanced by first priming with a pPyCSP and pGM-CSF mixture, followed by boosting with a recombinant poxvirus expressing PyCSP.[68] However, co-injection of plasmids encoding GM-CSF (or IFN-γ, or IL-2) and a fusion protein of P. chabaudi merozoite surface protein 1 (C-terminus)-hepatitis B virus surface protein (PcMSP1-HBs) abolished protection against challenge, compared to protection acquired by delivery of pPcMSP1-HBs alone.[30]
The advantages of genetic adjuvants are their low cost and simple administration, as well as avoidance of unstable recombinant cytokines and potentially toxic, "conventional" adjuvants (such as alum, calcium phosphate, monophosphoryl lipid A, cholera toxin, cationic and mannan-coated liposomes, QS21, carboxymethyl cellulose and ubenimex).[7][21] However, the potential toxicity of prolonged cytokine expression is not established. In many commercially important animal species, cytokine genes have not been identified and isolated. In addition, various plasmid-encoded cytokines modulate the immune system differently according to the delivery time. For example, some cytokine plasmid DNAs are best delivered after immunogen pDNA, because pre- or co-delivery can decrease specific responses and increase non-specific responses.[69]
Immunostimulatory CpG motifs
[edit]Plasmid DNA itself appears to have an adjuvant effect on the immune system.[6][7] Bacterially derived DNA can trigger innate immune defence mechanisms, the activation of dendritic cells and the production of TH1 cytokines.[45][70] This is due to recognition of certain CpG dinucleotide sequences that are immunostimulatory.[66][71] CpG stimulatory (CpG-S) sequences occur twenty times more frequently in bacterially-derived DNA than in eukaryotes. This is because eukaryotes exhibit "CpG suppression" – i.e. CpG dinucleotide pairs occur much less frequently than expected. Additionally, CpG-S sequences are hypomethylated. This occurs frequently in bacterial DNA, while CpG motifs occurring in eukaryotes are methylated at the cytosine nucleotide. In contrast, nucleotide sequences that inhibit the activation of an immune response (termed CpG neutralising, or CpG-N) are over represented in eukaryotic genomes.[72] The optimal immunostimulatory sequence is an unmethylated CpG dinucleotide flanked by two 5’ purines and two 3’ pyrimidines.[66][70] Additionally, flanking regions outside this immunostimulatory hexamer must be guanine-rich to ensure binding and uptake into target cells.
The innate system works with the adaptive immune system to mount a response against the DNA encoded protein. CpG-S sequences induce polyclonal B-cell activation and the upregulation of cytokine expression and secretion.[73] Stimulated macrophages secrete IL-12, IL-18, TNF-α, IFN-α, IFN-β and IFN-γ, while stimulated B-cells secrete IL-6 and some IL-12.[21][73][74]
Manipulation of CpG-S and CpG-N sequences in the plasmid backbone of DNA vaccines can ensure the success of the immune response to the encoded antigen and drive the immune response toward a TH1 phenotype. This is useful if a pathogen requires a TH response for protection. CpG-S sequences have also been used as external adjuvants for both DNA and recombinant protein vaccination with variable success rates. Other organisms with hypomethylated CpG motifs have demonstrated the stimulation of polyclonal B-cell expansion.[75] The mechanism behind this may be more complicated than simple methylation – hypomethylated murine DNA has not been found to mount an immune response.
Most of the evidence for immunostimulatory CpG sequences comes from murine studies. Extrapolation of this data to other species requires caution – individual species may require different flanking sequences, as binding specificities of scavenger receptors vary across species. Additionally, species such as ruminants may be insensitive to immunostimulatory sequences due to their large gastrointestinal load.
Alternative boosts
[edit]DNA-primed immune responses can be boosted by the administration of recombinant protein or recombinant poxviruses. "Prime-boost" strategies with recombinant protein have successfully increased both neutralising antibody titre, and antibody avidity and persistence, for weak immunogens, such as HIV-1 envelope protein.[7][76] Recombinant virus boosts have been shown to be very efficient at boosting DNA-primed CTL responses. Priming with DNA focuses the immune response on the required immunogen, while boosting with the recombinant virus provides a larger amount of expressed antigen, leading to a large increase in specific CTL responses.
Prime-boost strategies have been successful in inducing protection against malarial challenge in a number of studies. Primed mice with plasmid DNA encoding Plasmodium yoelii circumsporozoite surface protein (PyCSP), then boosted with a recombinant vaccinia virus expressing the same protein had significantly higher levels of antibody, CTL activity and IFN-γ, and hence higher levels of protection, than mice immunized and boosted with plasmid DNA alone.[77] This can be further enhanced by priming with a mixture of plasmids encoding PyCSP and murine GM-CSF, before boosting with recombinant vaccinia virus.[68] An effective prime-boost strategy for the simian malarial model P. knowlesi has also been demonstrated.[78] Rhesus monkeys were primed with a multicomponent, multistage DNA vaccine encoding two liver-stage antigens – the circumsporozoite surface protein (PkCSP) and sporozoite surface protein 2 (PkSSP2) – and two blood stage antigens – the apical merozoite surface protein 1 (PkAMA1) and merozoite surface protein 1 (PkMSP1p42). They were then boosted with a recombinant canarypox virus encoding all four antigens (ALVAC-4). Immunized monkeys developed antibodies against sporozoites and infected erythrocytes, and IFN-γ-secreting T-cell responses against peptides from PkCSP. Partial protection against sporozoite challenge was achieved, and mean parasitemia was significantly reduced, compared to control monkeys. These models, while not ideal for extrapolation to P. falciparum in humans, will be important in pre-clinical trials.
Enhancing immune responses
[edit]DNA
[edit]The efficiency of DNA immunization can be improved by stabilising DNA against degradation, and increasing the efficiency of delivery of DNA into antigen-presenting cells.[7] This has been demonstrated by coating biodegradable cationic microparticles (such as poly(lactide-co-glycolide) formulated with cetyltrimethylammonium bromide) with DNA. Such DNA-coated microparticles can be as effective at raising CTL as recombinant viruses, especially when mixed with alum. Particles 300 nm in diameter appear to be most efficient for uptake by antigen presenting cells.[7]
Alphavirus vectors
[edit]Recombinant alphavirus-based vectors have been used to improve DNA vaccination efficiency.[7] The gene encoding the antigen of interest is inserted into the alphavirus replicon, replacing structural genes but leaving non-structural replicase genes intact. The Sindbis virus and Semliki Forest virus have been used to build recombinant alphavirus replicons. Unlike conventional DNA vaccinations alphavirus vectors kill transfected cells and are only transiently expressed. Alphavirus replicase genes are expressed in addition to the vaccine insert. It is not clear how alphavirus replicons raise an immune response, but it may be due to the high levels of protein expressed by this vector, replicon-induced cytokine responses, or replicon-induced apoptosis leading to enhanced antigen uptake by dendritic cells.
See also
[edit]References
[edit]- ^ a b Henrique Roman Ramos and Paulo Lee Ho. "Developing Snake Antivenom Sera by Genetic Immunization: A Review". Clinical Toxinology in Asia Pacific and Africa. 2: 401–414.
- ^ a b Liu S, Wang S, Lu S (April 2016). "DNA immunization as a technology platform for monoclonal antibody induction". Emerging Microbes & Infections. 5 (4): e33. doi:10.1038/emi.2016.27. PMC 4855071. PMID 27048742.
- ^ a b Khan KH (March 2013). "DNA vaccines: roles against diseases". Germs. 3 (1): 26–35. doi:10.11599/germs.2013.1034. PMC 3882840. PMID 24432284.
- ^ a b "India gives emergency approval for world's first COVID-19 DNA vaccine". Reuters. 2021-08-20. Retrieved 2021-08-22.
- ^ a b c d e f g h i j k l m n Alarcon JB, Waine GW, McManus DP (1999). "DNA Vaccines: Technology and Application as Anti-parasite and Anti-microbial Agents". Advances in Parasitology Volume 42. Vol. 42. pp. 343–410. doi:10.1016/S0065-308X(08)60152-9. ISBN 9780120317424. PMID 10050276.
- ^ a b c d e f g h i j k l m n o p q r s t u v Robinson HL, Pertmer TM (2000). DNA vaccines for viral infections: basic studies and applications. Advances in Virus Research. Vol. 55. pp. 1–74. doi:10.1016/S0065-3527(00)55001-5. ISBN 9780120398553. PMID 11050940.
- ^ White LO, Gibb E, Newham HC, Richardson MD, Warren RC (July 1979). "Comparison of the growth of virulent and attenuated strains of Candida albicans in the kidneys of normal and cortison-treated mice by chitin assay". Mycopathologia. 67 (3): 173–177. doi:10.1007/bf00470753. PMID 384256. S2CID 31914107.
- ^ Paoletti E, Lipinskas BR, Samsonoff C, Mercer S, Panicali D (January 1984). "Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D". Proceedings of the National Academy of Sciences of the United States of America. 81 (1): 193–197. Bibcode:1984PNAS...81..193P. doi:10.1073/pnas.81.1.193. PMC 344637. PMID 6320164.
- ^ US Patent 4722848 - Method for immunizing animals with synthetically modified vaccinia virus
- ^ Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. (March 1993). "Heterologous protection against influenza by injection of DNA encoding a viral protein". Science. 259 (5102): 1745–1749. Bibcode:1993Sci...259.1745U. doi:10.1126/science.8456302. PMID 8456302.
- ^ Regalado A (2 August 2016). "The U.S. government has begun testing its first Zika vaccine in humans". MIT Technology Review Magazine. Retrieved 2016-08-06.
- ^ Chen Y, Wang S, Lu S (February 2014). "DNA Immunization for HIV Vaccine Development". Vaccines. 2 (1): 138–159. doi:10.3390/vaccines2010138. PMC 4494200. PMID 26344472.
- ^ Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, et al. (May 2011). "A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial". The Journal of Infectious Diseases. 203 (10): 1396–1404. doi:10.1093/infdis/jir054. PMC 3080891. PMID 21398392.
- ^ Kilpatrick AM, Dupuis AP, Chang GJ, Kramer LD (May 2010). "DNA vaccination of American robins (Turdus migratorius) against West Nile virus". Vector Borne and Zoonotic Diseases. 10 (4): 377–380. doi:10.1089/vbz.2009.0029. PMC 2883478. PMID 19874192.
- ^ a b Sedegah M, Hedstrom R, Hobart P, Hoffman SL (October 1994). "Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein". Proceedings of the National Academy of Sciences of the United States of America. 91 (21): 9866–9870. Bibcode:1994PNAS...91.9866S. doi:10.1073/pnas.91.21.9866. JSTOR 2365723. PMC 44918. PMID 7937907.
- ^ Harmon BT, Aly AE, Padegimas L, Sesenoglu-Laird O, Cooper MJ, Waszczak BL (May 2014). "Intranasal administration of plasmid DNA nanoparticles yields successful transfection and expression of a reporter protein in rat brain". Gene Therapy. 21 (5): 514–521. doi:10.1038/gt.2014.28. PMID 24670994. S2CID 5560134.
- ^ a b Mor G, Klinman DM, Shapiro S, Hagiwara E, Sedegah M, Norman JA, et al. (August 1995). "Complexity of the cytokine and antibody response elicited by immunizing mice with Plasmodium yoelii circumsporozoite protein plasmid DNA". Journal of Immunology. 155 (4): 2039–2046. doi:10.4049/jimmunol.155.4.2039. PMID 7636255. S2CID 37290980.
- ^ Leitner WW, Seguin MC, Ballou WR, Seitz JP, Schultz AM, Sheehy MJ, Lyon JA (December 1997). "Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites". Journal of Immunology. 159 (12): 6112–6119. doi:10.4049/jimmunol.159.12.6112. PMID 9550412. S2CID 37685499.
- ^ Böhm W, Kuhröber A, Paier T, Mertens T, Reimann J, Schirmbeck R (June 1996). "DNA vector constructs that prime hepatitis B surface antigen-specific cytotoxic T lymphocyte and antibody responses in mice after intramuscular injection". Journal of Immunological Methods. 193 (1): 29–40. doi:10.1016/0022-1759(96)00035-X. PMID 8690928.
- ^ a b c d e f g h i j k Lewis PJ, Babiuk LA (1999). DNA vaccines: a review. Vol. 54. Academic Press. pp. 129–88. doi:10.1016/S0065-3527(08)60367-X. ISBN 978-0-12-039854-6. PMID 10547676.
{{cite book}}:|journal=ignored (help) - ^ André S, Seed B, Eberle J, Schraut W, Bültmann A, Haas J (February 1998). "Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage". Journal of Virology. 72 (2): 1497–1503. doi:10.1128/JVI.72.2.1497-1503.1998. PMC 124631. PMID 9445053.
- ^ Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY, et al. (September 2003). "Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo". Virology. 314 (1): 134–146. doi:10.1016/S0042-6822(03)00459-8. PMID 14517067.
- ^ Oliveira PH, Prather KJ, Prazeres DM, Monteiro GA (September 2009). "Structural instability of plasmid biopharmaceuticals: challenges and implications". Trends in Biotechnology. 27 (9): 503–511. doi:10.1016/j.tibtech.2009.06.004. PMID 19656584.
- ^ Oliveira PH, Mairhofer J (September 2013). "Marker-free plasmids for biotechnological applications - implications and perspectives". Trends in Biotechnology. 31 (9): 539–547. doi:10.1016/j.tibtech.2013.06.001. PMID 23830144.
- ^ Kutzler MA, Weiner DB (October 2008). "DNA vaccines: ready for prime time?". Nature Reviews. Genetics. 9 (10): 776–788. doi:10.1038/nrg2432. PMC 4317294. PMID 18781156.
- ^ Rodriguez F, Zhang J, Whitton JL (November 1997). "DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction". Journal of Virology. 71 (11): 8497–8503. doi:10.1128/JVI.71.11.8497-8503.1997. PMC 192313. PMID 9343207.
- ^ a b Tobery TW, Siliciano RF (March 1997). "Targeting of HIV-1 antigens for rapid intracellular degradation enhances cytotoxic T lymphocyte (CTL) recognition and the induction of de novo CTL responses in vivo after immunization". The Journal of Experimental Medicine. 185 (5): 909–920. doi:10.1084/jem.185.5.909. PMC 2196169. PMID 9120397.
- ^ Huebener N, Fest S, Strandsby A, Michalsky E, Preissner R, Zeng Y, et al. (July 2008). "A rationally designed tyrosine hydroxylase DNA vaccine induces specific antineuroblastoma immunity". Molecular Cancer Therapeutics. 7 (7): 2241–2251. doi:10.1158/1535-7163.MCT-08-0109. PMID 18645033. S2CID 35652424.
- ^ a b Wunderlich G, Moura IC, del Portillo HA (October 2000). "Genetic immunization of BALB/c mice with a plasmid bearing the gene coding for a hybrid merozoite surface protein 1-hepatitis B virus surface protein fusion protects mice against lethal Plasmodium chabaudi chabaudi PC1 infection". Infection and Immunity. 68 (10): 5839–5845. doi:10.1128/IAI.68.10.5839-5845.2000. PMC 101545. PMID 10992493.
- ^ Weiner DB, Kennedy RC (July 1999). "Genetic vaccines". Scientific American. 281 (1): 50–57. Bibcode:1999SciAm.281a..50W. doi:10.1038/scientificamerican0799-50. PMID 10396782. Archived from the original on 2009-03-25. Retrieved 2007-11-21.
- ^ Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. (May 2000). "Increased DNA vaccine delivery and immunogenicity by electroporation in vivo". Journal of Immunology. 164 (9): 4635–4640. doi:10.4049/jimmunol.164.9.4635. PMID 10779767.
- ^ a b Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse BT (August 1997). "Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10". Journal of Immunology. 159 (4): 1945–1952. doi:10.4049/jimmunol.159.4.1945. PMID 9257860. S2CID 43203331.
- ^ a b Chen Y, Webster RG, Woodland DL (March 1998). "Induction of CD8+ T cell responses to dominant and subdominant epitopes and protective immunity to Sendai virus infection by DNA vaccination". Journal of Immunology. 160 (5): 2425–2432. doi:10.4049/jimmunol.160.5.2425. PMID 9498786. S2CID 2250871.
- ^ Lode HN, Huebener N, Zeng Y, Fest S, Weixler S, Gaedicke G (December 2004). "DNA minigene vaccination for adjuvant neuroblastoma therapy". Annals of the New York Academy of Sciences. 1028 (1): 113–121. Bibcode:2004NYASA1028..113L. doi:10.1196/annals.1322.012. PMID 15650237. S2CID 27240738.
- ^ Sizemore DR, Branstrom AA, Sadoff JC (October 1995). "Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization". Science. 270 (5234): 299–302. Bibcode:1995Sci...270..299S. doi:10.1126/science.270.5234.299. PMID 7569980. S2CID 12532901.
- ^ Nealon, Cory (25 November 2014). "A hybrid vehicle that delivers DNA". The State University of New York at Buffalo. Retrieved 16 December 2014.
- ^ Jones CH, Ravikrishnan A, Chen M, Reddinger R, Kamal Ahmadi M, Rane S, et al. (August 2014). "Hybrid biosynthetic gene therapy vector development and dual engineering capacity". Proceedings of the National Academy of Sciences of the United States of America. 111 (34): 12360–12365. Bibcode:2014PNAS..11112360J. doi:10.1073/pnas.1411355111. PMC 4151754. PMID 25114239.
- ^ Barry MA, Lai WC, Johnston SA (October 1995). "Protection against mycoplasma infection using expression-library immunization". Nature. 377 (6550): 632–635. Bibcode:1995Natur.377..632B. doi:10.1038/377632a0. PMID 7566175. S2CID 4306972.
- ^ Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL (December 1993). "DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations". Proceedings of the National Academy of Sciences of the United States of America. 90 (24): 11478–11482. Bibcode:1993PNAS...9011478F. doi:10.1073/pnas.90.24.11478. PMC 48007. PMID 8265577.
- ^ a b c d e Feltquate DM, Heaney S, Webster RG, Robinson HL (March 1997). "Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization". Journal of Immunology. 158 (5): 2278–2284. doi:10.4049/jimmunol.158.5.2278. PMID 9036975. S2CID 41368723.
- ^ a b c d Boyle CM, Morin M, Webster RG, Robinson HL (December 1996). "Role of different lymphoid tissues in the initiation and maintenance of DNA-raised antibody responses to the influenza virus H1 glycoprotein". Journal of Virology. 70 (12): 9074–9078. doi:10.1128/JVI.70.12.9074-9078.1996. PMC 191015. PMID 8971047.
- ^ Sällberg M, Townsend K, Chen M, O'Dea J, Banks T, Jolly DJ, et al. (July 1997). "Characterization of humoral and CD4+ cellular responses after genetic immunization with retroviral vectors expressing different forms of the hepatitis B virus core and e antigens". Journal of Virology. 71 (7): 5295–5303. doi:10.1128/JVI.71.7.5295-5303.1997. PMC 191766. PMID 9188598.
- ^ Banchereau J, Steinman RM (March 1998). "Dendritic cells and the control of immunity". Nature. 392 (6673): 245–252. Bibcode:1998Natur.392..245B. doi:10.1038/32588. PMID 9521319. S2CID 4388748.
- ^ a b Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC (September 1998). "Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA". Journal of Immunology. 161 (6): 3042–3049. doi:10.4049/jimmunol.161.6.3042. PMID 9743369. S2CID 35107733.
- ^ a b Raz E, Tighe H, Sato Y, Corr M, Dudler JA, Roman M, et al. (May 1996). "Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization". Proceedings of the National Academy of Sciences of the United States of America. 93 (10): 5141–5145. Bibcode:1996PNAS...93.5141R. doi:10.1073/pnas.93.10.5141. PMC 39421. PMID 8643542.
- ^ a b Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ (April 1997). "Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization". Journal of Virology. 71 (4): 2715–2721. doi:10.1128/JVI.71.4.2715-2721.1997. PMC 191393. PMID 9060624.
- ^ a b c Restifo NP, Bacík I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, et al. (May 1995). "Antigen processing in vivo and the elicitation of primary CTL responses". Journal of Immunology. 154 (9): 4414–4422. doi:10.4049/jimmunol.154.9.4414. PMC 1952186. PMID 7722298.
- ^ a b c Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH (May 1997). "Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines". Journal of Immunology. 158 (10): 4591–4601. doi:10.4049/jimmunol.158.10.4591. PMID 9144471. S2CID 41779568.
- ^ a b Weiss WR, Ishii KJ, Hedstrom RC, Sedegah M, Ichino M, Barnhart K, et al. (September 1998). "A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine". Journal of Immunology. 161 (5): 2325–2332. doi:10.4049/jimmunol.161.5.2325. PMID 9725227. S2CID 21038927.
- ^ Tsuji T, Hamajima K, Fukushima J, Xin KQ, Ishii N, Aoki I, et al. (April 1997). "Enhancement of cell-mediated immunity against HIV-1 induced by coinnoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12". Journal of Immunology. 158 (8): 4008–4013. doi:10.4049/jimmunol.158.8.4008. PMID 9103472. S2CID 38099483.
- ^ a b Justewicz DM, Webster RG (October 1996). "Long-term maintenance of B cell immunity to influenza virus hemagglutinin in mice following DNA-based immunization". Virology. 224 (1): 10–17. doi:10.1006/viro.1996.0501. PMID 8862394.
- ^ Mancini-Bourgine M, Fontaine H, Bréchot C, Pol S, Michel ML (May 2006). "Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers". Vaccine. 24 (21): 4482–4489. doi:10.1016/j.vaccine.2005.08.013. PMID 16310901.
- ^ a b Wolff JA, Dowty ME, Jiao S, Repetto G, Berg RK, Ludtke JJ, et al. (December 1992). "Expression of naked plasmids by cultured myotubes and entry of plasmids into T tubules and caveolae of mammalian skeletal muscle". Journal of Cell Science. 103. 103 (4): 1249–1259. doi:10.1242/jcs.103.4.1249. PMID 1487500.
- ^ Anderson RG, Kamen BA, Rothberg KG, Lacey SW (January 1992). "Potocytosis: sequestration and transport of small molecules by caveolae". Science. 255 (5043): 410–411. Bibcode:1992Sci...255..410A. doi:10.1126/science.1310359. PMID 1310359.
- ^ a b Casares S, Inaba K, Brumeanu TD, Steinman RM, Bona CA (November 1997). "Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope". The Journal of Experimental Medicine. 186 (9): 1481–1486. doi:10.1084/jem.186.9.1481. PMC 2199124. PMID 9348305.
- ^ a b Bennett RM, Gabor GT, Merritt MM (December 1985). "DNA binding to human leukocytes. Evidence for a receptor-mediated association, internalization, and degradation of DNA". The Journal of Clinical Investigation. 76 (6): 2182–2190. doi:10.1172/JCI112226. PMC 424340. PMID 3001145.
- ^ Bennet RM, Hefeneider SH, Bakke A, Merritt M, Smith CA, Mourich D, Heinrich MC (May 1988). "The production and characterization of murine monoclonal antibodies to a DNA receptor on human leukocytes". Journal of Immunology. 140 (9): 2937–2942. doi:10.4049/jimmunol.140.9.2937. PMID 2452195. S2CID 22923379.
- ^ Corr M, Lee DJ, Carson DA, Tighe H (October 1996). "Gene vaccination with naked plasmid DNA: mechanism of CTL priming". The Journal of Experimental Medicine. 184 (4): 1555–1560. doi:10.1084/jem.184.4.1555. PMC 2192808. PMID 8879229.
- ^ Chattergoon MA, Robinson TM, Boyer JD, Weiner DB (June 1998). "Specific immune induction following DNA-based immunization through in vivo transfection and activation of macrophages/antigen-presenting cells". Journal of Immunology. 160 (12): 5707–5718. doi:10.4049/jimmunol.160.12.5707. PMID 9637479. S2CID 33499198.
- ^ a b Torres CA, Iwasaki A, Barber BH, Robinson HL (May 1997). "Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations". Journal of Immunology. 158 (10): 4529–4532. doi:10.4049/jimmunol.158.10.4529. PMID 9144463. S2CID 45069087.
- ^ Franco A, Guidotti LG, Hobbs MV, Pasquetto V, Chisari FV (August 1997). "Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice". Journal of Immunology. 159 (4): 2001–2008. doi:10.4049/jimmunol.159.4.2001. PMID 9257867. S2CID 20528634.
- ^ Mancini M, Hadchouel M, Davis HL, Whalen RG, Tiollais P, Michel ML (October 1996). "DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state". Proceedings of the National Academy of Sciences of the United States of America. 93 (22): 12496–12501. Bibcode:1996PNAS...9312496M. doi:10.1073/pnas.93.22.12496. PMC 38020. PMID 8901610.
- ^ Doolan DL, Hoffman SL (July 1999). "IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model". Journal of Immunology. 163 (2): 884–892. doi:10.4049/jimmunol.163.2.884. PMID 10395683. S2CID 41651105.
- ^ Cardoso AI, Blixenkrone-Moller M, Fayolle J, Liu M, Buckland R, Wild TF (November 1996). "Immunization with plasmid DNA encoding for the measles virus hemagglutinin and nucleoprotein leads to humoral and cell-mediated immunity". Virology. 225 (2): 293–299. doi:10.1006/viro.1996.0603. PMID 8918915.
- ^ a b c Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, et al. (July 1996). "Immunostimulatory DNA sequences necessary for effective intradermal gene immunization". Science. 273 (5273): 352–354. Bibcode:1996Sci...273..352S. doi:10.1126/science.273.5273.352. PMID 8662521. S2CID 9333197.
- ^ Weiss R, Leitner WW, Scheiblhofer S, Chen D, Bernhaupt A, Mostböck S, et al. (October 2000). "Genetic vaccination against malaria infection by intradermal and epidermal injections of a plasmid containing the gene encoding the Plasmodium berghei circumsporozoite protein". Infection and Immunity. 68 (10): 5914–5919. doi:10.1128/IAI.68.10.5914-5919.2000. PMC 101554. PMID 10992502.
- ^ a b Sedegah M, Weiss W, Sacci JB, Charoenvit Y, Hedstrom R, Gowda K, et al. (June 2000). "Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus". Journal of Immunology. 164 (11): 5905–5912. doi:10.4049/jimmunol.164.11.5905. PMID 10820272.
- ^ Barouch DH, Santra S, Steenbeke TD, Zheng XX, Perry HC, Davies ME, et al. (August 1998). "Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration". Journal of Immunology. 161 (4): 1875–1882. doi:10.4049/jimmunol.161.4.1875. PMID 9712056. S2CID 36488254.
- ^ a b Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. (April 1995). "CpG motifs in bacterial DNA trigger direct B-cell activation". Nature. 374 (6522): 546–549. Bibcode:1995Natur.374..546K. doi:10.1038/374546a0. PMID 7700380. S2CID 4261304.
- ^ Klinman DM, Yamshchikov G, Ishigatsubo Y (April 1997). "Contribution of CpG motifs to the immunogenicity of DNA vaccines". Journal of Immunology. 158 (8): 3635–3639. doi:10.4049/jimmunol.158.8.3635. PMID 9103425. S2CID 41861994.
- ^ Krieg AM, Wu T, Weeratna R, Efler SM, Love-Homan L, Yang L, et al. (October 1998). "Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs". Proceedings of the National Academy of Sciences of the United States of America. 95 (21): 12631–12636. Bibcode:1998PNAS...9512631K. doi:10.1073/pnas.95.21.12631. PMC 22882. PMID 9770537.
- ^ a b Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM (April 1996). "CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma". Proceedings of the National Academy of Sciences of the United States of America. 93 (7): 2879–2883. Bibcode:1996PNAS...93.2879K. doi:10.1073/pnas.93.7.2879. PMC 39727. PMID 8610135.
- ^ Yi AK, Chace JH, Cowdery JS, Krieg AM (January 1996). "IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides". Journal of Immunology. 156 (2): 558–564. doi:10.4049/jimmunol.156.2.558. PMID 8543806. S2CID 42145608.
- ^ Barwick BG, Scharer CD, Martinez RJ, Price MJ, Wein AN, Haines RR, et al. (May 2018). "B cell activation and plasma cell differentiation are inhibited by de novo DNA methylation". Nature Communications. 9 (1): 1900. Bibcode:2018NatCo...9.1900B. doi:10.1038/s41467-018-04234-4. PMC 5953949. PMID 29765016.
- ^ Letvin NL, Montefiori DC, Yasutomi Y, Perry HC, Davies ME, Lekutis C, et al. (August 1997). "Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination". Proceedings of the National Academy of Sciences of the United States of America. 94 (17): 9378–9383. Bibcode:1997PNAS...94.9378L. doi:10.1073/pnas.94.17.9378. PMC 23198. PMID 9256490.
- ^ Sedegah M, Jones TR, Kaur M, Hedstrom R, Hobart P, Tine JA, Hoffman SL (June 1998). "Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine". Proceedings of the National Academy of Sciences of the United States of America. 95 (13): 7648–7653. Bibcode:1998PNAS...95.7648S. doi:10.1073/pnas.95.13.7648. PMC 22711. PMID 9636204.
- ^ Rogers WO, Baird JK, Kumar A, Tine JA, Weiss W, Aguiar JC, et al. (September 2001). "Multistage multiantigen heterologous prime boost vaccine for Plasmodium knowlesi malaria provides partial protection in rhesus macaques". Infection and Immunity. 69 (9): 5565–5572. doi:10.1128/IAI.69.9.5565-5572.2001. PMC 98670. PMID 11500430.
Further reading
[edit]- Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, et al. (May 2004). "Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox". Journal of Virology. 78 (9): 4433–4443. doi:10.1128/JVI.78.9.4433-4443.2004. PMC 387704. PMID 15078924.
