Dicarbonate
| |
| Names | |
|---|---|
| IUPAC name
Dicarbonate
| |
| Other names
Pyrocarbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| MeSH | pyrocarbonate |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2O2−5 | |
| Molar mass | 104.017 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |


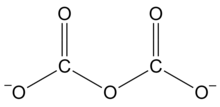
A dicarbonate, also known as a pyrocarbonate, is a chemical containing the divalent −O−C(=O)−O−C(=O)−O− or −C2O5− functional group, which consists of two carbonate groups sharing an oxygen atom. It is one of polycarbonate functional groups. These compounds can be viewed as derivatives of the hypothetical compound dicarbonic acid,[2] HO−C(=O)−O−C(=O)−OH or H2C2O5. Three important organic compounds containing this group are:
- dimethyl dicarbonate H3C−C2O5−CH3
- diethyl dicarbonate C2H5−C2O5−C2H5
- di-tert-butyl dicarbonate (H3C−)3C−C2O5−C(−CH3)3, also known as Boc anhydride.
It is one of the oxocarbon anions, consisting solely of oxygen and carbon. The anion has the formula −O−C(=O)−O−C(=O)−O− or C2O2−5. Dicarbonate salts are apparently unstable at ambient conditions, but can be made under pressure and may have a fleeting existence in carbonate solutions.[3]
The term dicarbonate is sometimes used erroneously to refer to bicarbonate, the common name of the hydrogencarbonate anion HCO−3 or esters of the hydrogencarbonate functional group −O−C(=O)−OH. It is also sometimes used for chemicals that contain two carbonate units in their covalent structure or stoichiometric formula.
Inorganic salts
[edit]PbC2O5 (lead(II) dicarbonate) can be formed at 30 GPa and 2000K from PbCO3 and CO2. It forms white monoclinic crystals, with space group P21/c and four formula units per unit cell. At 30 GPa the unit cell has a=4.771 b=8.079 c=7.070 Å and β=91.32°. The unit cell volume is 272.4 Å3 and density 7.59.[4]
SrC2O5 (strontium dicarbonate) is very similar to the lead compound, and also has monoclinic structure with space group P21/c and four formula units per unit cell. At 30 GPa the unit cell has a=4.736 b=8.175 c=7.140 Å and β=91.34°. The unit cell volume is 276.3 Å3 and density 4.61.[4] The double Sr=O bonds have lengths of 1.22, 1.24, and 1.25 Å. The single Sr-O bonds have lengths of 1.36 and 1.41 Å. The angles subtended at the carbon atoms are slightly less than 120°, and the angle at the C-O-C is larger.[5]
See also
[edit]- Tricarbonate
- Peroxodicarbonate
- Oxalate
- Pyrosulfate
- Peroxydisulfate
- Dithionate
- Trithionate
- Tetrathionate
- Pyrophosphate
- Polyphosphate
References
[edit]- ^ Plácido García; Helge Willner; Maximiliano Burgos Paci; Gustavo A. Argüello; Thorsten Berends (2005). "Bis(trifluoromethyl)dicarbonate, CF3OC(O)OC(O)OCF3". J. Fluorine Chem. 126 (6): 984–990. doi:10.1016/j.jfluchem.2005.05.002.
- ^ "CHEBI:48501 - dicarbonic acid". ChEBI. Retrieved 30 June 2024.
- ^ Zeller, Klaus-Peter; Schuler, Paul; Haiss, Peter (2005). "The hidden equilibrium in aqueous sodium carbonate solutions: Evidence for the formation of the dicarbonate anion". Eur. J. Inorg. Chem. 2005 (1): 168–172. doi:10.1002/ejic.200400445.
- ^ a b Spahr, Dominik; König, Jannes; Bayarjargal, Lkhamsuren; Luchitskaia, Rita; Milman, Victor; Perlov, Alexander; Liermann, Hanns-Peter; Winkler, Björn (22 June 2022). "Synthesis and Structure of Pb[C2O5]: An Inorganic Pyrocarbonate Salt". Inorganic Chemistry. 61 (26): 9855–9859. doi:10.1021/acs.inorgchem.2c01507. PMID 35730801. S2CID 249922854.
- ^ Spahr, Dominik; König, Jannes; Bayarjargal, Lkhamsuren; Milman, Victor; Perlov, Alexander; Liermann, Hanns-Peter; Winkler, Björn (23 February 2022). "Sr[C2O5 ] is an Inorganic Pyrocarbonate Salt with [C 2 O 5 ] 2– Complex Anions". Journal of the American Chemical Society. 144 (7): 2899–2904. doi:10.1021/jacs.2c00351. PMID 35134291. S2CID 246678040.
