From Wikipedia, the free encyclopedia
Chemical compound
Etynodiol Other names Ethynodiol; 3β-Hydroxynorethisterone; 17α-Ethynylestr-4-ene-3β,17β-diol Drug class Progestin ; Progestogen ATC code
(3S ,8R ,9S ,10R ,13S ,14S ,17R )-17-ethynyl-13-methyl-2,3,6,7,8,9,10,11,12,14,15,16-dodecahydro-1H -cyclopenta[a ]phenanthrene-3,17-diol
CAS Number ChemSpider UNII CompTox Dashboard (EPA ) ECHA InfoCard 100.013.610 Formula C 20 H 28 O 2 Molar mass −1 3D model (JSmol )
O[C@@H]4/C=C3\[C@@H]([C@H]2CC[C@]1([C@@H](CC[C@]1(C#C)O)[C@@H]2CC3)C)CC4
InChI=1S/C20H28O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,12,14-18,21-22H,4-11H2,2H3/t14-,15-,16+,17+,18-,19-,20-/m0/s1
Key:JYILPERKVHXLNF-QMNUTNMBSA-N
Etynodiol , or ethynodiol , is a steroidal progestin of the 19-nortestosterone group which was never marketed.[ 1] [ 2] [ 3] diacylated derivative, etynodiol diacetate , is used as a hormonal contraceptive .[ 1] [ 2] Etynodiol is sometimes used as a synonym for etynodiol diacetate .
It was patented in 1955.[ 4]
Etynodiol is a prodrug of norethisterone , and is converted immediately and completely into norethisterone.[ 5] [ 6] [ 7] intermediate in the conversion of the prodrug lynestrenol into norethisterone.[ 8]
Relative affinities (%) of norethisterone , metabolites, and prodrugs
Compound
Typea
PR Tooltip Progesterone receptor AR Tooltip Androgen receptor ER Tooltip Estrogen receptor GR Tooltip Glucocorticoid receptor MR Tooltip Mineralocorticoid receptor SHBG Tooltip Sex hormone-binding globulin CBG Tooltip Corticosteroid binding globulin
Norethisterone –
67–75
15
0
0–1
0–3
16
0
5α-Dihydronorethisterone Metabolite
25
27
0
0
?
?
?
3α,5α-Tetrahydronorethisterone
Metabolite
1
0
0–1
0
?
?
?
3α,5β-Tetrahydronorethisterone
Metabolite
?
0
0
?
?
?
?
3β,5α-Tetrahydronorethisterone
Metabolite
1
0
0–8
0
?
?
?
Ethinylestradiol Metabolite
15–25
1–3
112
1–3
0
0.18
0
Norethisterone acetate Prodrug
20
5
1
0
0
?
?
Norethisterone enanthate Prodrug
?
?
?
?
?
?
?
Noretynodrel Prodrug
6
0
2
0
0
0
0
Etynodiol
Prodrug
1
0
11–18
0
?
?
?
Etynodiol diacetate Prodrug
1
0
0
0
0
?
?
Lynestrenol Prodrug
1
1
3
0
0
?
?
Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR Tooltip progesterone receptor , metribolone for the AR Tooltip androgen receptor , estradiol for the ER Tooltip estrogen receptor , dexamethasone for the GR Tooltip glucocorticoid receptor , aldosterone for the MR Tooltip mineralocorticoid receptor , dihydrotestosterone for SHBG Tooltip sex hormone-binding globulin , and cortisol for CBG Tooltip Corticosteroid-binding globulin . Footnotes: a = Active or inactive metabolite , prodrug , or neither of norethisterone. Sources: See template.
Etynodiol is a 19-nortestosterone derivative. Structurally, it is almost identical to norethisterone and lynestrenol , differing only in its C3 substituent. Whereas norethisterone has a ketone at C3 and lynestrenol has no substituent at C3, etynodiol has a hydroxyl group at the position.
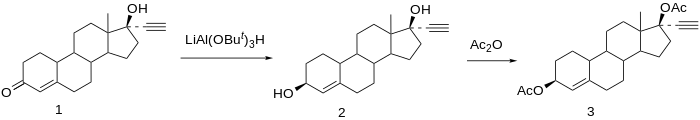
Ethynodiol diacetate synthesis:[ 9] U.S. patent 2,843,609 Searle ). Prepn of the 3-acetate, 17-acetate, and diacetate: P. D. Klimstra, U.S. patent 3,176,013 [ 10]
Society and culture [ edit ] Etynodiol is the generic name of the drug and its INN Tooltip International Nonproprietary Name , while ethynodiol is its BAN Tooltip British Approved Name .[ 1] [ 2]
^ a b c Macdonald F (1997). Dictionary of Pharmacological Agents ISBN 978-0-412-46630-4 . Retrieved 12 May 2012 . ^ a b c Index Nominum 2000: International Drug Directory ISBN 978-3-88763-075-1 . Retrieved 30 May 2012 .^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins". Maturitas . 46 (Suppl 1): S7–S16. doi :10.1016/j.maturitas.2003.09.014 . PMID 14670641 . ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery ISBN 9783527607495 ^ Shoupe D, Haseltine FP (6 December 2012). Contraception ISBN 978-1-4612-2730-4 ^ Bhattacharya (1 January 2003). Pharmacology, 2/e ISBN 978-81-8147-009-6 ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy ISBN 978-92-832-1291-1 ^ Hammerstein J (December 1990). "Prodrugs: advantage or disadvantage?". American Journal of Obstetrics and Gynecology . 163 (6 Pt 2): 2198–203. doi :10.1016/0002-9378(90)90561-K . PMID 2256526 . ^ Klimstra PD, Colton FB (October 1967). "The synthesis of 3beta-hydroxyestr-4-en-17-one and 3beta-hydroxyandrost-4-en-17-one". Steroids . 10 (4): 411–24. doi :10.1016/0039-128X(67)90119-5 . PMID 6064262 . ^ Sondheimer F, Klibansky Y (1959). "Synthesis of 3β-hydroxy analogues of steroidal hormones, a biologically active class of compounds". Tetrahedron . 5 : 15–26. doi :10.1016/0040-4020(59)80066-1 .
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
AR Tooltip Androgen receptor
Agonists SARMs Tooltip Selective androgen receptor modulator Antagonists
GPRC6A