Censavudine
Appearance
(Redirected from Festinavir)
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4'-ethynylstavudine, festinavir |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.225.812 |
| Chemical and physical data | |
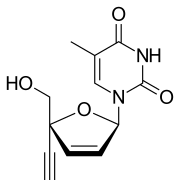
| Formula | C12H12N2O4 |
| Molar mass | 248.238 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Censavudine (INN;[1] development code BMS-986001) is an investigational new drug being developed by Bristol Myers-Squibb for the treatment of HIV infection.[2][3] It was originally developed at Yale University.[4] It is still in an investigational phase of development as of 2023.[5]
Renaming
[edit]Until 2013, censavudine has been known as festinavir, but the name was changed to avoid confusion with HIV protease inhibitors which all bear class suffix "–navir" (e.g. tipranavir, lopinavir, saquinavir, etc.).[5]
References
[edit]- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed International Nonproprietary Names: List 110" (PDF). World Health Organization. pp. 409–410.
- ^ Wu VH, Smith RA, Masoum S, Raugi DN, Ba S, Seydi M, et al. (August 2017). "MK-8591 (4'-Ethynyl-2-Fluoro-2'-Deoxyadenosine) Exhibits Potent Activity against HIV-2 Isolates and Drug-Resistant HIV-2 Mutants in Culture". Antimicrobial Agents and Chemotherapy. 61 (8). doi:10.1128/AAC.00744-17. PMC 5527656. PMID 28559249.
- ^ Gupta SK, McComsey GA, Lombaard J, Echevarría J, Orrell C, Avihingsanon A, et al. (January 2016). "Efficacy, safety, bone and metabolic effects of HIV nucleoside reverse transcriptase inhibitor BMS-986001 (AI467003): a phase 2b randomised, controlled, partly blinded trial". The Lancet. HIV. 3 (1): e13-22. doi:10.1016/S2352-3018(15)00231-3. hdl:1805/10552. PMID 26762988.
- ^ Alcorn K (21 December 2010). "Bristol-Myers Squibb buys festinavir, new NRTI active against MDR HIV". aidsmap.com. aidsmap. Retrieved 24 June 2011.
- ^ a b PubChem. "Censavudine". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-10-24.
