Phenylketonuria
| Phenylketonuria | |
|---|---|
| Other names | Phenylalanine hydroxylase deficiency, PAH deficiency, Følling disease[1] |
 | |
| Phenylalanine | |
| Specialty | Medical genetics, pediatrics, dietetics |
| Symptoms | Without treatment intellectual disability, seizures, behavioral problems, mental disorders, musty odor[1] |
| Usual onset | At birth[2] |
| Types | Classic, variant[1] |
| Causes | Genetic (autosomal recessive)[1] |
| Diagnostic method | Newborn screening programs in many countries[3] |
| Treatment | Diet low in foods that contain phenylalanine; special supplements[2] |
| Medication | Sapropterin dihydrochloride,[2] pegvaliase[4] |
| Prognosis | Normal health with treatment[5] |
| Frequency | ~1 in 12,000 newborns[6] |
Phenylketonuria (PKU) is an inborn error of metabolism that results in decreased metabolism of the amino acid phenylalanine.[3] Untreated PKU can lead to intellectual disability, seizures, behavioral problems, and mental disorders.[1][7] It may also result in a musty smell and lighter skin.[1] A baby born to a mother who has poorly treated PKU may have heart problems, a small head, and low birth weight.[1]
Phenylketonuria is an inherited genetic disorder. It is caused by mutations in the PAH gene, which can result in inefficient or nonfunctional phenylalanine hydroxylase, an enzyme responsible for the metabolism of excess phenylalanine. This results in the buildup of dietary phenylalanine to potentially toxic levels. It is autosomal recessive, meaning that both copies of the gene must be mutated for the condition to develop. There are two main types, classic PKU and variant PKU, depending on whether any enzyme function remains. Those with one copy of a mutated gene typically do not have symptoms.[1] Many countries have newborn screening programs for the disease.[3]
Treatment is with a diet that (1) is low in foods that contain phenylalanine, and which (2) includes special supplements. Babies should use a special formula with a small amount of breast milk. The diet should begin as soon as possible after birth and be continued for life.[2] People who are diagnosed early and maintain a strict diet can have normal health and a normal life span. Effectiveness is monitored through periodic blood tests.[5] The medication sapropterin dihydrochloride may be useful in some.[2]
Phenylketonuria affects about 1 in 12,000 babies.[6] Males and females are affected equally.[8] The disease was discovered in 1934 by Ivar Asbjørn Følling, with the importance of diet determined in 1935.[9] As of 2023, genetic therapies that aim to directly restore liver PAH activity are a promising and active research field.[10]
Signs and symptoms
[edit]
Untreated PKU can lead to intellectual disability, seizures, behavioral problems, and mental disorders. It may also result in a musty smell and lighter skin. A baby born to a mother who has poorly treated PKU may have heart problems, a small head, and low birth weight.[1]
Because the mother's body is able to break down phenylalanine during pregnancy, infants with PKU are normal at birth. The disease is not detectable by physical examination at that time, because no damage has yet been done. Newborn screening is performed to detect the disease and initiate treatment before any damage is done. The blood sample is usually taken by a heel prick, typically performed 2–7 days after birth. This test can reveal elevated phenylalanine levels after one or two days of normal infant feeding.[11][12]
If a child is not diagnosed during the routine newborn screening test and a phenylalanine-restricted diet is not introduced, then phenylalanine levels in the blood will increase over time. Toxic levels of phenylalanine (and insufficient levels of tyrosine) can interfere with infant development in ways that have permanent effects. The disease may present clinically with seizures, hypopigmentation (excessively fair hair and skin), and a "musty odor" to the baby's sweat and urine (due to phenylacetate, a carboxylic acid produced by the oxidation of phenylacetone). In most cases, a repeat test should be done at approximately two weeks of age to verify the initial test and uncover any phenylketonuria that was initially missed.[13]
Untreated children often fail to attain early developmental milestones, develop microcephaly, and demonstrate progressive impairment of cerebral function. Hyperactivity, EEG abnormalities, and seizures, and severe learning disabilities are major clinical problems later in life. A characteristic "musty or mousy" odor on the skin, as well as a predisposition for eczema, persist throughout life in the absence of treatment.[14]
The damage done to the brain if PKU is untreated during the first months of life is not reversible. It is critical to control the diet of infants with PKU very carefully so that the brain has an opportunity to develop normally. Affected children who are detected at birth and treated are much less likely to develop neurological problems or have seizures and intellectual disability (though such clinical disorders are still possible including asthma, eczema, anemia, weight gain, renal insufficiency, osteoporosis, gastritis, esophagus, and kidney deficiencies, kidney stones, and hypertension). Additionally, major depressive disorders occur 230% higher than controls; dizziness and giddiness occur 180% higher; chronic ischemic heart disease, asthma, diabetes, and gastroenteritis occur 170% higher; and stress and adjustment disorders occur 160% higher.[15][16] In general, however, outcomes for people treated for PKU are good. Treated people may have no detectable physical, neurological, or developmental problems at all.[citation needed]
Genetics
[edit]
PKU is an autosomal recessive metabolic genetic disorder. As an autosomal recessive disorder, two PKU alleles are required for an individual to experience symptoms of the disease. For a child to inherit PKU, both parents must have and pass on the defective gene.[17] If both parents are carriers for PKU, there is a 25% chance any child they have will be born with the disorder, a 50% chance the child will be a carrier and a 25% chance the child will neither develop nor be a carrier for the disease.[5]
PKU is characterized by homozygous or compound heterozygous mutations in the gene for the hepatic enzyme phenylalanine hydroxylase (PAH), rendering it nonfunctional.[18]: 541 This enzyme is necessary to metabolize the amino acid phenylalanine (Phe) to the amino acid tyrosine (Tyr). When PAH activity is reduced, phenylalanine accumulates and is converted into phenylpyruvate (also known as phenylketone), which can be detected in the urine.[19]
Carriers of a single PKU allele do not exhibit symptoms of the disease but appear to be protected to some extent against the fungal toxin ochratoxin A. Louis Woolf suggested that this accounted for the persistence of the allele in certain populations,[20] in that it confers a selective advantage—in other words, being a heterozygote is advantageous.[21]
The PAH gene is located on chromosome 12 in the bands 12q22-q24.2.[22] As of 2000, around 400 disease-causing mutations had been found in the PAH gene. This is an example of allelic genetic heterogeneity.[5]
Pathophysiology
[edit]When phenylalanine (Phe) cannot be metabolized by the body, a typical diet that would be healthy for people without PKU causes abnormally high levels of Phe to accumulate in the blood, which is toxic to the brain. If left untreated (and often even in treatment), complications of PKU include severe intellectual disability, brain function abnormalities, microcephaly, mood disorders, irregular motor functioning, and behavioral problems such as attention deficit hyperactivity disorder, as well as physical symptoms such as a "musty" odor, eczema, and unusually light skin and hair coloration.[23]
Classical PKU
[edit]Classical PKU, and its less severe forms "mild PKU" and "mild hyperphenylalaninemia" are caused by a mutated gene for the enzyme phenylalanine hydroxylase (PAH), which converts the amino acid phenylalanine ("Phe") to other essential compounds in the body, in particular tyrosine. Tyrosine is a conditionally essential amino acid for PKU patients because without PAH it cannot be produced in the body through the breakdown of phenylalanine.[citation needed]
PAH deficiency causes a spectrum of disorders, including classic phenylketonuria (PKU) and mild hyperphenylalaninemia (also known as "hyperphe" or "mild HPA"),[24] a less severe accumulation of phenylalanine. Compared to classic PKU patients, patients with "hyperphe" have greater PAH enzyme activity and are able to tolerate larger amounts of phenylalanine in their diets. Without dietary intervention, mild HPA patients have blood Phe levels higher than those with normal PAH activity. There is currently no international consensus on the definition of mild HPA, however, it is most frequently diagnosed at blood Phe levels between 2–6 mg/dL.[25]
Phenylalanine is a large, neutral amino acid (LNAA). LNAAs compete for transport across the blood–brain barrier (BBB) via the large neutral amino acid transporter (LNAAT). If phenylalanine is in excess in the blood, it will saturate the transporter. Excessive levels of phenylalanine tend to decrease the levels of other LNAAs in the brain. As these amino acids are necessary for protein and neurotransmitter synthesis, Phe buildup hinders the development of the brain, causing intellectual disability.[26]
Recent research suggests that neurocognitive, psychosocial, quality of life, growth, nutrition, bone pathology are slightly suboptimal even for patients who are treated and maintain their Phe levels in the target range, if their diet is not supplemented with other amino acids.[27]
Classic PKU affects myelination and white matter tracts in untreated infants; this may be one major cause of neurological problems associated with phenylketonuria. Differences in white matter development are observable with magnetic resonance imaging. Abnormalities in gray matter can also be detected,[28] particularly in the motor and pre-motor cortex, thalamus and the hippocampus.[29]
It was recently suggested that PKU may resemble amyloid diseases, such as Alzheimer's disease and Parkinson's disease, due to the formation of toxic amyloid-like assemblies of phenylalanine.[30]
Tetrahydrobiopterin-deficient hyperphenylalaninemia
[edit]A rarer form of hyperphenylalaninemia is tetrahydrobiopterin deficiency, which occurs when the PAH enzyme is normal, and a defect is found in the biosynthesis or recycling of the cofactor tetrahydrobiopterin (BH4).[31] BH4 is necessary for proper activity of the enzyme PAH, and this coenzyme can be supplemented as treatment. Those with this form of hyperphenylalaninemia may have a deficiency of tyrosine (which is created from phenylalanine by PAH), in which case treatment is supplementation of tyrosine to account for this deficiency.[citation needed]
Levels of dopamine can be used to distinguish between these two types. Tetrahydrobiopterin is required to convert Phe to Tyr and is required to convert Tyr to L-DOPA via the enzyme tyrosine hydroxylase. L-DOPA, in turn, is converted to dopamine. Low levels of dopamine lead to high levels of prolactin. By contrast, in classical PKU (without dihydrobiopterin involvement), prolactin levels would be relatively normal.[32][citation needed]
As of 2020, tetrahydrobiopterin deficiency was known to result from defects in five genes.[33]
Metabolic pathways
[edit]
The enzyme phenylalanine hydroxylase normally converts the amino acid phenylalanine into the amino acid tyrosine. If this reaction does not take place, phenylalanine accumulates and tyrosine is deficient. Excessive phenylalanine can be metabolized into phenylketones through the minor route, a transaminase pathway with glutamate. Metabolites include phenylacetate, phenylpyruvate and phenethylamine.[34] Elevated levels of phenylalanine in the blood and detection of phenylketones in the urine is diagnostic, however most patients are diagnosed via newborn screening.[citation needed][35]
Screening
[edit]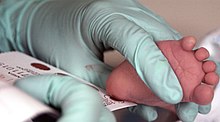
PKU is commonly included in the newborn screening panel of many countries, with varied detection techniques. Most babies born in Europe, North America, and Australia are screened for PKU soon after birth.[36][37] Screening for PKU is done with bacterial inhibition assay (Guthrie test), immunoassays using fluorometric or photometric detection, or amino acid measurement using tandem mass spectrometry (MS/MS). Measurements done using MS/MS determine the concentration of Phe and the ratio of Phe to tyrosine, the ratio will be elevated in PKU.[38]
Treatment
[edit]PKU is not curable. However, if it is diagnosed early enough, an affected newborn can grow up with normal brain development by managing and controlling phenylalanine ("Phe") levels through diet, or a combination of diet and medication.[39] If dietary treatment is not initiated within 2 weeks after birth, the child is likely to develop permanent intellectual disability, even if dietary interventions begin shortly thereafter.[37]
Diet
[edit]People who follow the prescribed dietary treatment from birth may (but not always) have no symptoms. Their PKU would be detectable only by a blood test. People must adhere to a special diet low in Phe for optimal brain development. Since Phe is necessary for the synthesis of many proteins, it is required for appropriate growth, but levels must be strictly controlled.[37]

Optimal health ranges (or "target ranges") are between 120 and 360 μmol/L or equivalently 2 to 6 mg/dL. This is optimally to be achieved during at least the first 10 years,[40] to allow the brain to develop normally.[citation needed]
The diet requires restricting or eliminating foods high in Phe, such as soybeans, egg whites, shrimp, chicken breast, spirulina, watercress, fish, nuts, crayfish, lobster, tuna, turkey, legumes, and lowfat cottage cheese.[41] Starchy foods, such as potatoes and corn are generally acceptable in controlled amounts, but the quantity of Phe consumed from these foods must be monitored. A corn-free diet may be prescribed in some cases. A food diary is usually kept to record the amount of Phe consumed with each meal, snack, or drink. An "exchange" system can be used to calculate the amount of Phe in a portion of food from the protein content identified on a nutritional information label. Lower-protein "medical food" substitutes are often used in place of normal bread, pasta, and other grain-based foods, which contain a significant amount of Phe. Many fruits and vegetables are lower in Phe and can be eaten in larger quantities. Infants may still be breastfed to provide all of the benefits of breastmilk, but the quantity must also be monitored and supplementation for missing nutrients will be required. The sweetener aspartame, present in many diet foods and soft drinks, must also be avoided, as aspartame contains phenylalanine.[42]
The amino acid tyrosine becomes essential in people with phenylalanine hydroxylase deficiency. Thus, in addition to the careful reduction of Phe in the diet, Tyr must be supplemented to ensure that nutritional needs are met.[37]
Different people can tolerate different amounts of Phe in their diet. Regular blood tests are used to determine the effects of dietary Phe intake on blood Phe level.[citation needed]
Nutritional supplements
[edit]Supplementary "protein substitute" formulas are typically prescribed for people PKU (starting in infancy) to provide the amino acids and other necessary nutrients that would otherwise be lacking in a low-phenylalanine diet. Tyrosine, which is normally derived from phenylalanine and which is necessary for normal brain function, is usually supplemented. Consumption of the protein substitute formulas can actually reduce phenylalanine levels, probably because it stops the process of protein catabolism from releasing Phe stored in the muscles and other tissues into the blood. Many PKU patients have their highest Phe levels after a period of fasting (such as overnight) because fasting triggers catabolism.[43] A diet that is low in phenylalanine but does not include protein substitutes may also fail to lower blood Phe levels, since a nutritionally insufficient diet may also trigger catabolism. For all these reasons, the prescription formula is an important part of the treatment for patients with classic PKU.[citation needed]
Evidence supports dietary supplementation with large neutral amino acids (LNAAs).[44] The LNAAs (e.g. leu, tyr, trp, met, his, ile, val, thr) may compete with phe for specific carrier proteins that transport LNAAs across the intestinal mucosa into the blood and across the blood–brain barrier into the brain. Its use is limited in the US due to the cost but is available in most countries as part of a low protein / PHE diet to replace missing nutrients.[citation needed]
Another treatment strategy is casein glycomacropeptide (CGMP), which is a milk peptide naturally free of Phe in its pure form[45] CGMP can substitute for the main part of the free amino acids in the PKU diet and provides several beneficial nutritional effects compared to free amino acids. The fact that CGMP is a peptide ensures that the absorption rate of its amino acids is prolonged compared to free amino acids and thereby results in improved protein retention[46] and increased satiety[47] compared to free amino acids. Another important benefit of CGMP is that the taste is significantly improved[46] when CGMP substitutes part of the free amino acids and this may help ensure improved compliance to the PKU diet.[citation needed]
Furthermore, CGMP contains a high amount of the Phe-lowering LNAAs, which constitutes about 41 g per 100 g protein[45] and will therefore help maintain plasma Phe levels in the target range.
Enzyme substitutes
[edit]In 2018, the FDA approved an enzyme substitute called pegvaliase which metabolizes phenylalanine.[4] It is for adults who are poorly managed on other treatments.[4]
Tetrahydrobiopterin (BH4) (a cofactor for the oxidation of phenylalanine) when taken by mouth can reduce blood levels of this amino acid in some people.[48][49]
Mothers
[edit]For women with PKU, it is important for the health of their children to maintain low Phe levels before and during pregnancy.[50] Though the developing fetus may only be a carrier of the PKU gene, the intrauterine environment can have very high levels of phenylalanine, which can cross the placenta. The child may develop congenital heart disease, growth retardation, microcephaly and intellectual disability as a result.[51] PKU-affected women themselves are not at risk of additional complications during pregnancy.[citation needed]
In most countries, women with PKU who wish to have children are advised to lower their blood Phe levels (typically to between 2 and 6 mg/dL) before they become pregnant, and carefully control their levels throughout the pregnancy. This is achieved by performing regular blood tests and adhering very strictly to a diet, in general monitored on a day-to-day basis by a specialist metabolic dietitian. In many cases, as the fetus' liver begins to develop and produce PAH normally, the mother's blood Phe levels will drop, requiring an increased intake to remain within the safe range of 2–6 mg/dL. The mother's daily Phe intake may double or even triple by the end of the pregnancy, as a result. When maternal blood Phe levels fall below 2 mg/dL, anecdotal reports indicate that the mothers may experience adverse effects, including headaches, nausea, hair loss, and general malaise. When low phenylalanine levels are maintained for the duration of pregnancy, there are no elevated levels of risk of birth defects compared with a baby born to a non-PKU mother.[52]
Epidemiology
[edit]| Country | Incidence |
|---|---|
| Australia | 1 in 10,000[53] |
| Brazil | 1 in 8,690 |
| Canada | 1 in 22,000[53] |
| China | 1 in 17,000[53] |
| Czechoslovakia | 1 in 7,000[53] |
| Denmark | 1 in 12,000[53] |
| Finland | 1 in 200,000[53] |
| France | 1 in 13,500[53] |
| India | 1 in 18,300 |
| Ireland | 1 in 4,500[54] |
| Italy | 1 in 17,000[53] |
| Japan | 1 in 125,000[53] |
| Korea | 1 in 41,000[55] |
| Netherlands | 1 in 18,000[56] |
| Norway | 1 in 14,500[53] |
| Philippines | 1 in 102,000[57] |
| Poland | 1 in 8,000[56] |
| Scotland | 1 in 5,300[53] |
| Spain | 1 in 20,000[56] |
| Sweden | 1 in 20,000[56] |
| Turkey | 1 in 2,600[53] |
| United Kingdom | 1 in 10,000[56] |
| United States | 1 in 25,000[58] |
The average number of new cases of PKU varies in different human populations. United States Caucasians are affected at a rate of 1 in 10,000.[59] Turkey has the highest documented rate in the world, with 1 in 2,600 births, while countries such as Finland and Japan have extremely low rates with fewer than one case of PKU in 100,000 births. A 1987 study from Slovakia reports a Roma population with an extremely high incidence of PKU (one case in 40 births) due to extensive inbreeding.[60] It is the most common amino acid metabolic problem in the United Kingdom.[citation needed]
History
[edit]Before the causes of PKU were understood, PKU caused severe disability in most people who inherited the relevant mutations. Nobel and Pulitzer Prize winning author Pearl S. Buck had a daughter named Carol who lived with PKU before treatment was available, and wrote an account of its effects in a book called The Child Who Never Grew.[61] Many untreated PKU patients born before widespread newborn screening are still alive, largely in dependent living homes/institutions.[62]
Phenylketonuria was discovered by the Norwegian physician Ivar Asbjørn Følling in 1934[63] when he noticed hyperphenylalaninemia (HPA) was associated with intellectual disability. In Norway, this disorder is known as Følling's disease, named after its discoverer.[64] Følling was one of the first physicians to apply detailed chemical analysis to the study of disease.[citation needed]
In 1934 at Rikshospitalet, Følling saw a young woman named Borgny Egeland. She had two children, Liv and Dag, who had been normal at birth but subsequently developed intellectual disability. When Dag was about a year old, the mother noticed a strong smell to his urine. Følling obtained urine samples from the children and, after many tests, he found that the substance causing the odor in the urine was phenylpyruvic acid. The children, he concluded, had excess phenylpyruvic acid in the urine, the condition which came to be called phenylketonuria (PKU).[19]
His analysis of the urine of the two affected siblings led him to request many physicians near Oslo to test the urine of other affected patients. This led to the discovery of the same substance he had found in eight other patients. He conducted tests and found reactions that gave rise to benzaldehyde and benzoic acid, which led him to conclude that the compound contained a benzene ring. Further testing showed the melting point to be the same as phenylpyruvic acid, which indicated that the substance was in the urine.[65]
In 1954, Horst Bickel, Evelyn Hickmans and John Gerrard published a paper that described how they created a diet that was low in phenylalanine and the patient recovered. Bickel, Gerrard and Hickmans were awarded the John Scott Medal in 1962 for their discovery.[66]
PKU was the first disorder to be routinely diagnosed through widespread newborn screening. Robert Guthrie introduced the newborn screening test for PKU in the early 1960s.[67] With the knowledge that PKU could be detected before symptoms were evident, and treatment initiated, screening was quickly adopted around the world. Ireland was the first country to introduce a national screening programme in February 1966,[68] Austria also started screening in 1966[69] and England in 1968.[70]
In 2017, the European Guidelines were published.[71] They were called for by the patient organizations such as the European Society for Phenylketonuria and Allied Disorders Treated as Phenylketonuria.[72][73] They have received some critical reception.[74]
Etymology and pronunciation
[edit]The word phenylketonuria uses combining forms of phenyl + ketone + -uria; it is pronounced /ˌfiːnaɪlˌkiːtəˈnjʊəriə, ˌfɛn-, -nɪl-, -nəl-, -toʊ-/[75][76].
Research
[edit]Other therapies are under investigation, including gene therapy.
BioMarin is conducting clinical trials to investigate PEG-PAL (PEGylated recombinant phenylalanine ammonia lyase or 'PAL'), which is an enzyme substitution therapy in which the missing PAH enzyme is replaced with an analogous enzyme that also breaks down Phe. PEG-PAL was in Phase 2 clinical development as of 2015,[77] but was put on clinical hold in September 2021. In February 2022, the FDA issued a statement requiring further data from non-clinical studies to assess oncogenic risk resulting from PEG-PAL treatments.[78]
See also
[edit]- Hyperphenylalanemia
- Lofenalac
- Tetrahydrobiopterin deficiency
- Flowers for Algernon, which features a character who has PKU.
- 1968 Film Charly, a movie version of Flowers for Algernon
References
[edit]- ^ a b c d e f g h i "phenylketonuria". Genetics Home Reference. September 8, 2016. Archived from the original on 27 July 2016. Retrieved 12 September 2016.
- ^ a b c d e "What are common treatments for phenylketonuria (PKU)?". NICHD. 2013-08-23. Archived from the original on 5 October 2016. Retrieved 12 September 2016.
- ^ a b c Al Hafid N, Christodoulou J (October 2015). "Phenylketonuria: a review of current and future treatments". Translational Pediatrics. 4 (4): 304–17. doi:10.3978/j.issn.2224-4336.2015.10.07. PMC 4728993. PMID 26835392.
- ^ a b c "Press Announcements - FDA approves a new treatment for PKU, a rare and serious genetic disease". www.fda.gov. Retrieved 9 December 2018.
- ^ a b c d "National Institutes of Health Consensus Development Conference Statement Phenylketonuria: Screening and Management". NICHD. October 16–18, 2000. Archived from the original on 5 October 2016. Retrieved 12 September 2016.
- ^ a b Bernstein LE, Rohr F, Helm JR (2015). Nutrition Management of Inherited Metabolic Diseases: Lessons from Metabolic University. Springer. p. 91. ISBN 9783319146218. Archived from the original on 2017-09-11.
- ^ Cannon Homaei S, Barone H, Kleppe R, Betari N, Reif A, Haavik J (November 2021). "ADHD symptoms in neurometabolic diseases: Underlying mechanisms and clinical implications". Neuroscience and Biobehavioral Reviews. 132: 838–856. doi:10.1016/j.neubiorev.2021.11.012. PMID 34774900. S2CID 243983688.
- ^ Marcdante K, Kliegman RM (2014). Nelson Essentials of Pediatrics (7 ed.). Elsevier Health Sciences. p. 150. ISBN 9780323226981. Archived from the original on 2017-09-11.
- ^ Kalter H (2010). Teratology in the Twentieth Century Plus Ten. Springer Science & Business Media. pp. 89–92. ISBN 9789048188208. Archived from the original on 2017-09-11.
- ^ Martinez M, Harding CO, Schwank G, Thöny B (January 2024). "State-of-the-art 2023 on gene therapy for phenylketonuria". Journal of Inherited Metabolic Disease. 47 (1): 80–92. doi:10.1002/jimd.12651. ISSN 0141-8955. PMC 10764640. PMID 37401651.
- ^ "Phenylketonuria (PKU) Test". HealthLink BC. Archived from the original on May 17, 2018. Retrieved Aug 28, 2020.
- ^ Berry SA, Brown C, Grant M, Greene CL, Jurecki E, Koch J, Moseley K, Suter R, van Calcar SC, Wiles J, Cederbaum S (August 2013). "Newborn screening 50 years later: access issues faced by adults with PKU". Genetics in Medicine. 15 (8): 591–9. doi:10.1038/gim.2013.10. PMC 3938172. PMID 23470838.
- ^ "Phenylketonuria (PKU)". Madriella Doula Network. Madriella Network. 14 October 2016. Retrieved 11 April 2021.
- ^ "Phenylketonuria". MarkerDB. Wishart Research Group. Retrieved 11 April 2021.
- ^ Burton BK, Jones KB, Cederbaum S, Rohr F, Waisbren S, Irwin DE, et al. (2018). "Prevalence of comorbid conditions among adult patients diagnosed with phenylketonuria". Mol Genet Metab. 125 (3): 228–234. doi:10.1016/j.ymgme.2018.09.006. PMID 30266197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Trefz KF, Muntau AC, Kohlscheen KM, Altevers J, Jacob C, Braun S, et al. (2019). "Clinical burden of illness in patients with phenylketonuria (PKU) and associated comorbidities - a retrospective study of German health insurance claims data". Orphanet J Rare Dis. 14 (1): 181. doi:10.1186/s13023-019-1153-y. PMC 6647060. PMID 31331350.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Phenylketonuria (PKU) - Symptoms and causes". Mayo Clinic. Retrieved 2020-03-10.
- ^ James WD, Berger TG (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6.
- ^ a b Gonzalez J, Willis MS (Feb 2010). "Ivar Asbjörn Følling". Laboratory Medicine. 41 (2): 118–119. doi:10.1309/LM62LVV5OSLUJOQF.
- ^ Woolf LI (May 1986). "The heterozygote advantage in phenylketonuria". American Journal of Human Genetics. 38 (5): 773–5. PMC 1684820. PMID 3717163.
- ^ Lewis R (1997). Human Genetics. Chicago, IL: Wm. C. Brown. pp. 247–248. ISBN 978-0-697-24030-9.
- ^ Rosenberg RN, Barchi RL, DiMauro S, Prusiner SB, Nestler EJ (2003). The Molecular and Genetic Basis of Neurologic and Psychiatric Disease. Butterworth-Heinemann. p. 820. ISBN 9780750673600.
- ^ Ashe K, Kelso W, Farrand S, Panetta J, Fazio T, De Jong G, Walterfang M (2019). "Psychiatric and Cognitive Aspects of Phenylketonuria: The Limitations of Diet and Promise of New Treatments". Front. Psychiatry. 10 (561): 561. doi:10.3389/fpsyt.2019.00561. PMC 6748028. PMID 31551819.
- ^ Regier DS, Greene CL (July 25, 1993). "Phenylalanine Hydroxylase Deficiency". In Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). GeneReviews®. University of Washington, Seattle. PMID 20301677 – via PubMed.
- ^ de la Parra A, García MI, Waisbren SE, Cornejo V, Raimann E (December 2015). "Cognitive functioning in mild hyperphenylalaninemia". Molecular Genetics and Metabolism Reports. 5: 72–75. doi:10.1016/j.ymgmr.2015.10.009. PMC 5471391. PMID 28649547.
- ^ Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ (1999). "Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria". Journal of Clinical Investigation. 103 (8): 1169–1178. doi:10.1172/JCI5017. PMC 408272. PMID 10207169.
- ^ Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E (1 October 2010). "Suboptimal outcomes in patients with PKU treated early with diet alone: Revisiting the evidence". Molecular Genetics and Metabolism. 101 (2–3): 99–109. doi:10.1016/j.ymgme.2010.05.017. PMID 20678948.
- ^ Terribilli D, Schaufelberger MS (10 May 2020). "Age-related gray matter volume changes in the brain during non-elderly adulthood". Neurobiology of Aging. 32 (2–6): 354–368. doi:10.1016/j.neurobiolaging.2009.02.008. PMC 3004040. PMID 19282066.
- ^ Hawks Z, Hood AM, Lerman-Sinkoff DB, Shimony JS, Rutlin J, Lagoni D, Grange DK, White DA (2019-01-01). "White and gray matter brain development in children and young adults with phenylketonuria". NeuroImage: Clinical. 23: 101916. doi:10.1016/j.nicl.2019.101916. ISSN 2213-1582. PMC 6627563. PMID 31491833.
- ^ Adler-Abramovich L, Vaks L, Carny O, Trudler D, Magno A, Caflisch A, Frenkel D, Gazit E (August 2012). "Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria". Nature Chemical Biology. 8 (8): 701–6. doi:10.1038/nchembio.1002. PMID 22706200.
- ^ Surtees R, Blau N (2000). "The neurochemistry of phenylketonuria". European Journal of Pediatrics. 169: S109–S113. doi:10.1007/PL00014370. PMID 11043156. S2CID 26196359.
- ^ Opladen T, López-Laso E (26 May 2020). "Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies". Orphanet Journal of Rare Diseases. 15 (1): 126. doi:10.1186/s13023-020-01379-8. PMC 7251883. PMID 32456656.
- ^ Opladen T, López-Laso E, Cortès-Saladelafont E, Pearson TS, Sivri HS, Yildiz Y, Assmann B, Kurian MA, Leuzzi V, Heales S, Pope S, Porta F, García-Cazorla A, Honzík T, Pons R, Regal L, Goez H, Artuch R, Hoffmann GF, Horvath G, Thöny B, Scholl-Bürgi S, Burlina A, Verbeek MM, Mastrangelo M, Friedman J, Wassenberg T, Jeltsch K, Kulhánek J, Kuseyri Hübschmann O (May 2020). "Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies". Orphanet Journal of Rare Diseases. 15 (1): 126. doi:10.1186/s13023-020-01379-8. PMC 7251883. PMID 32456656.
- ^ Michals K, Matalon R (1985). "Phenylalanine metabolites, attention span and hyperactivity". American Journal of Clinical Nutrition. 42 (2): 361–5. doi:10.1093/ajcn/42.2.361. PMID 4025205.
- ^ "Phenylketonuria (PKU) - Diagnosis". Mayo Clinic. Retrieved 18 July 2024.
- ^ Mayo Clinic Staff (2007-12-20). "Phenylketonuria (PKU)". Mayo Clinic. Archived from the original on 2008-03-17. Retrieved 2008-03-13.
- ^ a b c d Longo N (2022). "Chapter 420: Inherited Disorders of Amino Acid Metabolism in Adults". Harrison's Principles of Internal Medicine (21st ed.). New York: McGraw Hill.
- ^ Sarafoglou K, Hoffmann GF, Roth KS (eds.). Pediatric Endocrinology and Inborn Errors of Metabolism. New York: McGraw Hill Medical. p. 26.
- ^ Widaman KF (2009-02-01). "Phenylketonuria in Children and Mothers: Genes, Environments, Behavior". Current Directions in Psychological Science. 18 (1): 48–52. doi:10.1111/j.1467-8721.2009.01604.x. ISSN 0963-7214. PMC 2705125. PMID 20126294.
- ^ Chapter 55, page 255 Archived 2016-05-11 at the Wayback Machine in:Behrman, Richard E., Kliegman, Robert, Nelson, Waldo E., Karen Marcdante, Jenson, Hal B. (2006). Nelson essentials of pediatrics. Elsevier/Saunders. ISBN 978-1-4160-0159-1.
- ^ "Foods highest in Phenylalanine". self.com. Archived from the original on 2015-05-05.
- ^ "CFR – Code of Federal Regulations, Title 21, Part 172: Food additives permitted for direct addition to food for human consumption. Subpart I – Multipurpose Additives; Sec. 172.804 Aspartame". US Food and Drug Administration. 1 April 2018. Retrieved 22 August 2019.
- ^ MacDonald A, Rylance GW, Asplin D, Hall SK, Booth IW (1998). "Does a single plasma phenylalanine predict the quality of control in phenylketonuria?". Archives of Disease in Childhood. 78 (2): 122–6. doi:10.1136/adc.78.2.122. PMC 1717471. PMID 9579152.
- ^ van Spronsen FJ, de Groot MJ, Hoeksma M, Reijngoud DJ, van Rijn M (December 2010). "Large neutral amino acids in the treatment of PKU: from theory to practice". Journal of Inherited Metabolic Disease. 33 (6): 671–6. doi:10.1007/s10545-010-9216-1. PMC 2992655. PMID 20976625.
- ^ a b Etzel MR (Apr 2004). "Manufacture and use of dairy protein fractions". The Journal of Nutrition. 134 (4): 996S–1002S. doi:10.1093/jn/134.4.996S. PMID 15051860.
- ^ a b van Calcar SC, MacLeod EL, Gleason ST, Etzel MR, Clayton MK, Wolff JA, Ney DM (Apr 2009). "Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids". The American Journal of Clinical Nutrition. 89 (4): 1068–77. doi:10.3945/ajcn.2008.27280. PMC 2667457. PMID 19244369.
- ^ MacLeod EL, Clayton MK, van Calcar SC, Ney DM (August 2010). "Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria". Molecular Genetics and Metabolism. 100 (4): 303–8. doi:10.1016/j.ymgme.2010.04.003. PMC 2906609. PMID 20466571.
- ^ Burton BK, Kar S, Kirkpatrick P (2008). "Sapropterin". Nature Reviews Drug Discovery. 7 (3): 199–200. doi:10.1038/nrd2540. S2CID 263991793.
- ^ Michals-Matalon K (February 2008). "Sapropterin dihydrochloride, 6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, in the treatment of phenylketonuria". Expert Opinion on Investigational Drugs. 17 (2): 245–51. doi:10.1517/13543784.17.2.245. PMID 18230057. S2CID 207475494.
- ^ Lee PJ, Ridout D, Walter JH, Cockburn F (2005). "Maternal phenylketonuria: report from the United Kingdom Registry 1978–97". Archives of Disease in Childhood. 90 (2): 143–146. doi:10.1136/adc.2003.037762. PMC 1720245. PMID 15665165.
- ^ Rouse B, Azen C, Koch R, Matalon R, Hanley W, de la Cruz F, Trefz F, Friedman E, Shifrin H (1997). "Maternal phenylketonuria collaborative study (MPKUCS) offspring: Facial anomalies, malformations, and early neurological sequelae". American Journal of Medical Genetics. 69 (1): 89–95. doi:10.1002/(SICI)1096-8628(19970303)69:1<89::AID-AJMG17>3.0.CO;2-K. PMID 9066890.
- ^ lsuhsc.edu Archived 2008-04-08 at the Wayback Machine Genetics and Louisiana Families
- ^ a b c d e f g h i j k l Williams RA, Mamotte CD, Burnett JR (February 2008). "Phenylketonuria: an inborn error of phenylalanine metabolism". The Clinical Biochemist. Reviews. 29 (1): 31–41. PMC 2423317. PMID 18566668.
- ^ DiLella AG, Kwok SC, Ledley FD, Marvit J, Woo SL (1986). "Molecular structure and polymorphic map of the human phenylalanine hydroxylase gene". Biochemistry. 25 (4): 743–749. doi:10.1021/bi00352a001. PMID 3008810.
- ^ Lee DH, Koo SK, Lee KS, Yeon YJ, Oh HJ, Kim SW, Lee SJ, Kim SS, Lee JE, Jo I, Jung SC (2004). "The molecular basis of phenylketonuria in Koreans". Journal of Human Genetics. 49 (1): 617–621. doi:10.1007/s10038-004-0197-5. PMID 15503242. S2CID 21446773.
- ^ a b c d e "PKU: Closing the Gaps in Care" (PDF). Retrieved Aug 28, 2020.
- ^ "Philippine Society for Orphan Disorders – Current Registry". psod.org.ph. Archived from the original on 2015-01-04.
- ^ Phenylketonuria at eMedicine
- ^ Bickel, H., Bachmann, C., Beckers, R., Brandt, N.J., Clayton, B.E., Corrado, G, et al. (1981). "Neonatal mass screening for metabolic disorders". European Journal of Pediatrics. 137 (137): 133–139. doi:10.1007/BF00441305. S2CID 44705699.
- ^ Ferák V, Siváková D, Sieglová Z (1987). "Slovenskí Cigáni (Rómovia) – populácia s najvyšším koeficientom inbrídingu v Európe". Bratislavské Lekárske Listy. 87 (2): 168–175.
- ^ Borg C, Mondot S, Mestre M, Cavero I (November 1991). "Nicorandil: differential contribution of K+ channel opening and guanylate cyclase stimulation to its vasorelaxant effects on various endothelin-1-contracted arterial preparations. Comparison to aprikalim (RP 52891) and nitroglycerin". The Journal of Pharmacology and Experimental Therapeutics. 259 (2): 526–34. PMID 1682478.
- ^ "NPKUA > Education > About PKU". npkua.org. Archived from the original on 2015-01-01.
- ^ Følling A (1 January 1934). "Über Ausscheidung von Phenylbrenztraubensäure in den Harn als Stoffwechselanomalie in Verbindung mit Imbezillität". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 227 (1–4): 169–181. doi:10.1515/bchm2.1934.227.1-4.169.
- ^ Centerwall SA, Centerwall WR (2000). "The discovery of phenylketonuria: the story of a young couple, two affected children, and a scientist". Pediatrics. 105 (1 Pt 1): 89–103. doi:10.1542/peds.105.1.89. PMID 10617710. S2CID 35922780.
- ^ Williams RA, Mamotte CD, Burnett JR (2008). "Phenylketonuria: an inborn error of phenylalanine metabolism". The Clinical Biochemist Reviews. 29 (1): 31–41. PMC 2423317. PMID 18566668.
Mild oxidation of the purified substance produced a compound which smelled of benzoic acid, leading Følling to postulate that the compound was phenylpyruvic acid.3 There was no change in the melting point upon mixing of the unknown compound with phenylpyruvic acid thus confirming the mystery compound was indeed phenylpyruvic acid.
- ^ Marelene Rayner-Canham, Geoff Rayner-Canham (2008), "Evelyn Hickmans", Chemistry was Their Life: Pioneer British Women Chemists, 1880–1949, World Scientific, p. 198, ISBN 9781908978998
- ^ Mitchell JJ, Trakadis YJ, Scriver CR (2011). "Phenylalanine hydroxylase deficiency". Genetics in Medicine. 13 (8): 697–707. doi:10.1097/GIM.0b013e3182141b48. PMID 21555948. S2CID 25921607.
- ^ Koch J (1997). Robert Guthrie--the PKU story : crusade against mental retardation. Pasadena, Calif.: Hope Pub. House. pp. 65–66. ISBN 0932727913. OCLC 36352725.
- ^ Kasper DC, Ratschmann R, Metz TF, Mechtler TP, Möslinger D, Konstantopoulou V, Item CB, Pollak A, Herkner KR (2010). "The National Austrian Newborn Screening Program – Eight years experience with mass spectrometry. Past, present, and future goals". Wiener Klinische Wochenschrift. 122 (21–22): 607–613. doi:10.1007/s00508-010-1457-3. PMID 20938748. S2CID 27643449.
- ^ Komrower GM, Sardharwalla IB, Fowler B, Bridge C (1979). "The Manchester regional screening programme: A 10-year exercise in patient and family care". British Medical Journal. 2 (6191): 635–638. doi:10.1136/bmj.2.6191.635. PMC 1596331. PMID 497752.
- ^ van Wegberg AM, MacDonald A, Ahring K, Bélanger-Quintana A, Blau N, Bosch AM, Burlina A, Campistol J, Feillet F, Giżewska M, Huijbregts SC, Kearney S, Leuzzi V, Maillot F, Muntau AC, van Rijn M, Trefz F, Walter JH, van Spronsen FJ (October 2017). "The complete European guidelines on phenylketonuria: diagnosis and treatment". Orphanet Journal of Rare Diseases. 12 (1): 162. doi:10.1186/s13023-017-0685-2. PMC 5639803. PMID 29025426.
- ^ "Consensus Paper - E.S.PKU". E.S.PKU. Retrieved 2018-11-23.
- ^ Hagedorn TS, van Berkel P, Hammerschmidt G, Lhotáková M, Saludes RP (December 2013). "Requirements for a minimum standard of care for phenylketonuria: the patients' perspective". Orphanet Journal of Rare Diseases. 8 (1): 191. doi:10.1186/1750-1172-8-191. PMC 3878574. PMID 24341788.
- ^ Burgard P, Ullrich K, Ballhausen D, Hennermann JB, Hollak CE, Langeveld M, Karall D, Konstantopoulou V, Maier EM, Lang F, Lachmann R, Murphy E, Garbade S, Hoffmann GF, Kölker S, Lindner M, Zschocke J (September 2017). "Issues with European guidelines for phenylketonuria". The Lancet. Diabetes & Endocrinology. 5 (9): 681–683. doi:10.1016/S2213-8587(17)30201-2. PMID 28842158.
- ^ "Phenylketonuria". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ "Phenylketonuria". Lexico UK English Dictionary. Oxford University Press. Archived from the original on 2020-08-05.
- ^ "BioMarin : Pipeline : Pipeline Overview : BMN 165 for PKU". bmrn.com. Archived from the original on 2015-01-01.
- ^ "BioMarin Provides Updates on Progress in Gene Therapy Programs". BioMarin Investors. Retrieved 2023-09-04.
