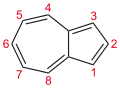
Azulene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azulene[1] | |||
| Systematic IUPAC name
Bicyclo[5.3.0]decapentaene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.449 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H8 | |||
| Molar mass | 128.174 g·mol−1 | ||
| Melting point | 99 to 100 °C (210 to 212 °F; 372 to 373 K) | ||
| Boiling point | 242 °C (468 °F; 515 K) | ||
| -98.5·10−6 cm3/mol
g/L[2] | |||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−1266.5 kcal/mol[3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates.
Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile. The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse. Its structure was first reported by Lavoslav Ružička, followed by its organic synthesis in 1937 by Placidus Plattner.
Structure and bonding
[edit]

Azulene is usually viewed as resulting from fusion of cyclopentadiene and cycloheptatriene rings. Like naphthalene and cyclodecapentaene, it is a 10 pi electron system. It exhibits aromatic properties: (i) the peripheral bonds have similar lengths and (ii) it undergoes Friedel-Crafts-like substitutions. The stability gain from aromaticity is estimated to be half that of naphthalene.
Its dipole moment is 1.08 D,[6] in contrast with naphthalene, which has a dipole moment of zero. This polarity can be explained by regarding azulene as the fusion of a 6 π-electron cyclopentadienyl anion and a 6 π-electron tropylium cation: one electron from the seven-membered ring is transferred to the five-membered ring to give each ring aromatic stability by Hückel's rule. Reactivity studies confirm that seven-membered ring is electrophilic and the five-membered ring is nucleophilic.

The dipolar nature of the ground state is reflected in its deep colour, which is unusual for small unsaturated aromatic compounds.[7] Another notable feature of azulene is that it violates Kasha's rule by exhibiting fluorescence from an upper-excited state (S2 → S0).[8]
Organic synthesis
[edit]Synthetic routes to azulene have long been of interest because of its unusual structure.[9] In 1939 the first method was reported by St. Pfau and Plattner [10] starting from indane and ethyl diazoacetate.
An efficient one-pot route entails annulation of cyclopentadiene with unsaturated C5-synthons.[11] The alternative approach from cycloheptatriene has long been known, one illustrative method being shown below.[12][13]
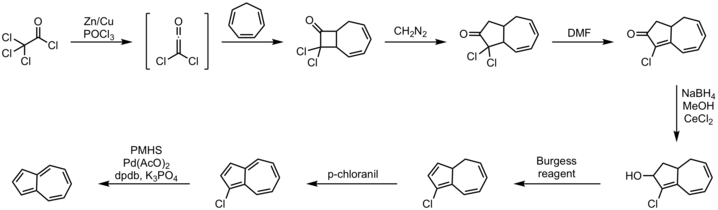
Procedure:
- cycloheptatriene 2+2 cycloaddition with dichloro ketene
- diazomethane insertion reaction
- dehydrohalogenation reaction with DMF
- Luche reduction to alcohol with sodium borohydride
- elimination reaction with Burgess reagent
- oxidation with p-chloranil
- dehalogenation with polymethylhydrosiloxane, palladium(II) acetate, potassium phosphate and the DPDB ligand
Another synthesis route starts from the of pyridinium or pyrylium salts with cyclopentadienyl anion:[14]

Azulene can also be synthesized via a Diels Alder and retro-Diels Alder reaction:[14]

The starting material of the above reaction can be generated through the Flash Vacuum Pyrolysis of phenyl propiolate.
Organometallic complexes
[edit]In organometallic chemistry, azulene serves as a ligand for low-valent metal centers. Illustrative complexes are (azulene)Mo2(CO)6 and (azulene)Fe2(CO)5.[15]
Derivatives
[edit]1-Hydroxyazulene is an unstable green oil and it does not show keto–enol tautomerism.[16] 2-Hydroxyazulene is obtained by hydrolysis of 2-methoxyazulene with hydrobromic acid. It is stable and does show keto–enol tautomerism.[17] The pKa of 2-hydroxyazulene in water is 8.71. It is more acidic than phenol or naphthol. The pKa of 6-hydroxyazulenes in water is 7.38 making it also more acidic than phenol or naphthol.[17]
In naphth[a]azulene, a naphthalene ring is condensed at the 1,2-positions of azulene. In one such system[18] deviation from planarity is found, similar to that of tetrahelicene.
Guaiazulene (1,4-dimethyl-7-isopropylazulene) is an alkylated derivative of azulene with an almost identical intensely blue colour. It is commercially available to the cosmetics industry where it functions as a skin conditioning agent.
References
[edit]- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 207. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Sweet, L. I.; Meier, P. G. (1997). "Lethal and Sublethal Effects of Azulene and Longifolene to Microtox®, Ceriodaphnia dubia, Daphnia magna, and Pimephales promelas" (PDF). Bulletin of Environmental Contamination and Toxicology. 58 (2): 268–274. Bibcode:1997BuECT..58..268S. doi:10.1007/s001289900330. hdl:2027.42/42354. PMID 8975804.
- ^ Salter, Carl; Foresman, James B. (1998). "Naphthalene and Azulene I: Semimicro Bomb Calorimetry and Quantum Mechanical Calculations". Journal of Chemical Education. 75 (10): 1341. Bibcode:1998JChEd..75.1341S. doi:10.1021/ed075p1341.
- ^ Harmon, A. D.; Weisgraber, K. H.; Weiss, U. (1980). "Preformed azulene pigments of Lactarius indigo (Schw.) Fries (Russulaceae, Basidiomycetes)". Experientia. 36: 54–56. doi:10.1007/BF02003967. S2CID 21207966.
- ^ Nicholas, Gillian May (1998). Australasian fungi: a natural product study (Thesis). p. 56. doi:10.26021/9162.
- ^ Anderson, Arthur G.; Steckler, Bernard M. (1959). "Azulene. VIII. A Study of the Visible Absorption Spectra and Dipole Moments of Some 1- and 1,3-Substituted Azulenes". Journal of the American Chemical Society. 81 (18): 4941–4946. doi:10.1021/ja01527a046.
- ^ Michl, Joseph; Thulstrup, E. W. (1976). "Why is azulene blue and anthracene white? A simple mo picture". Tetrahedron. 32 (2): 205. doi:10.1016/0040-4020(76)87002-0.
- ^ Tétreault, N.; Muthyala, R. S.; Liu, R. S. H.; Steer, R.P. (1999). "Control of the Photophysical Properties of Polyatomic Molecules by Substitution and Solvation: The Second Excited Singlet State of Azulene". Journal of Physical Chemistry A. 103 (15): 2524–31. Bibcode:1999JPCA..103.2524T. doi:10.1021/jp984407q.
- ^ Gordon, Maxwell (1 February 1952). "The Azulenes". Chemical Reviews. 50 (1): 127–200. doi:10.1021/cr60155a004.
- ^ St. Pfau, Alexander; Plattner, Pl. A. (1939). "Zur Kenntnis der flüchtigen Pflanzenstoffe VIII. Synthese des Vetivazulens". Helvetica Chimica Acta. 22: 202–208. doi:10.1002/hlca.19390220126.
- ^ Hafner, Klaus; Meinhardt, Klaus-Peter (1984). "Azulene". Organic Syntheses. 62: 134. doi:10.15227/orgsyn.062.0134.
- ^ Carret, Sébastien; Blanc, Aurélien; Coquerel, Yoann; Berthod, Mikaël; Greene, Andrew E.; Deprés, Jean-Pierre (2005). "Approach to the Blues: A Highly Flexible Route to the Azulenes". Angewandte Chemie International Edition. 44 (32): 5130–5133. doi:10.1002/anie.200501276. PMID 16013070.
- ^ Lemal, David M.; Goldman, Glenn D. (1988). "Synthesis of azulene, a blue hydrocarbon". Journal of Chemical Education. 65 (10): 923. Bibcode:1988JChEd..65..923L. doi:10.1021/ed065p923.
- ^ a b Shoji, Taku; Ito, Shunji; Yasunami, Masafumi (1 October 2021). "Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]furan-2-ones as Starting Materials: Their Reactivity and Properties". International Journal of Molecular Sciences. 22 (19): 10686. doi:10.3390/ijms221910686. ISSN 1422-0067. PMC 8509482. PMID 34639027.
- ^ Churchill, Melvyn R. (2007). "Transition Metal Complexes of Azulene and Related Ligands". Progress in Inorganic Chemistry. Vol. 11. pp. 53–98. doi:10.1002/9780470166123.ch2. ISBN 9780470166123.
- ^ Asao, Toyonobu; Shunji Ito; Noboru Morita (1989). "1-Hydroxyazulene and 3-hydroxyguaiazulene: Synthesis and their properties". Tetrahedron Letters. 30 (48): 6693–6696. doi:10.1016/S0040-4039(00)70653-8.
- ^ a b Takase, Kahei; Toyonobu Asao; Yoshikazu Takagi; Tetsuo Nozoe (1968). "Syntheses and some properties of 2- and 6-hydroxyazulenes". Chemical Communications (7): 368b–370. doi:10.1039/C1968000368B.
- ^ Yamamura, Kimiaki; Kawabata, Shizuka; Kimura, Takatomo; Eda, Kazuo; Hashimoto, Masao (2005). "Novel Synthesis of Benzalacetone Analogues of Naphth[a]azulenes by Intramolecular Tropylium Ion-Mediated Furan Ring-Opening Reaction and X-ray Investigation of a Naphth[1,2-a]azulene Derivative". The Journal of Organic Chemistry. 70 (22): 8902–6. doi:10.1021/jo051409f. PMID 16238325.