Jelly-falls

Jelly-falls are marine carbon cycling events whereby gelatinous zooplankton, primarily cnidarians, sink to the seafloor and enhance carbon and nitrogen fluxes via rapidly sinking particulate organic matter.[1] These events provide nutrition to benthic megafauna and bacteria.[2][3] Jelly-falls have been implicated as a major “gelatinous pathway” for the sequestration of labile biogenic carbon through the biological pump.[4] These events are common in protected areas with high levels of primary production and water quality suitable to support cnidarian species. These areas include estuaries and several studies have been conducted in fjords of Norway.[3]
Initiation
[edit]| Part of a series on the |
| Carbon cycle |
|---|
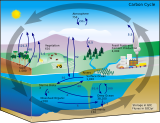 |
Jelly-falls are primarily made up of the decaying corpses of Cnidaria and Thaliacea (Pyrosomida, Doliolida, and Salpida).[1] Several circumstances can trigger the death of gelatinous organisms which would cause them to sink. These include high levels of primary production that can clog the feeding apparatuses of the organisms, a sudden temperature change, when an old bloom runs out of food, when predators damage the bodies of the jellies, and parasitism.[5] In general, however, jelly-falls are linked to jelly-blooms and primary production, with over 75% of the jelly falls in subpolar and temperate regions occurring after spring blooms, and over 25% of the jelly-falls in the tropics occurring after upwelling events.[1]
With global climates shifting towards creating warmer and more acidic oceans, conditions not favored by non-resilient species, jellies are likely to grow in population sizes. Eutrophic areas and dead zones can become jelly hot spots with substantial blooms.[6] As the climate changes and ocean waters warm, jelly blooms become more prolific and the transport of jelly-carbon to the lower ocean increases.[7] With a possible slowing of the classic biological pump, the transport of carbon and nutrients to the deep sea through jelly-falls may become more and more important to deep ocean.[8]
Decomposition
[edit]The decomposition process starts after death and can proceed in the water column as the gelatinous organisms are sinking.[5] Decay happens faster in the tropics than in temperate and subpolar waters as a result of warmer temperatures.[5] In the tropics, a jelly-fall may take less than 2 days to decay in warmer, surface water, but as many as 25 days when it is lower than 1000 m deep.[5] However, lone gelatinous organisms may spend less time on the sea floor as one study found that jellies could be decomposed by scavengers in the Norwegian deep sea in under two and a half hours.[9]
Decomposition of jelly-falls is largely aided by these kinds of scavengers. In general, echinoderms, such as sea stars, have emerged as the primary consumer of jelly-falls, followed by crustaceans and fish.[1] However, which scavengers find their way to jelly-falls is highly reliant on each ecosystem. For example, in an experiment in the Norwegian deep sea, hagfish were the first scavengers to find the traps of decaying jellies, followed by squat lobsters, and finally decapod shrimp.[9] Photographs taken off the coast of Norway on natural jelly-falls also revealed caridean shrimp feeding on jelly carcasses.[3]
With increased populations and blooms becoming more common, with favorable conditions and a lack of other filter feeders in the area to consume plankton, environments with jellies present will have carbon pumps be more primarily supplied with jelly-falls. This could lead to issues of habitats with established biological pumps succumbing to nonequilibrium as the presence of jellies would change the food web as well as changes to the amount of carbon deposited into the sediment.[10] Finally, decomposition is aided by the microbial community. In a case study on the Black Sea, the number of bacteria increased in the presence of jelly-falls, and the bacteria were shown to preferentially use nitrogen released from decaying jelly carcasses while mostly leaving carbon.[11] In a study conducted by Andrew Sweetman in 2016, it was discovered using core samples of the sediment in Norwegian fjords, the presence of jelly-falls significantly impacted the biochemical process of these benthic communities. Bacteria consume jelly carcasses rapidly, removing opportunities of acquiring sustenance for bottoming feeding macrofauna, which has impacts traveling up the trophic levels.[12] In addition, with the exclusion of scavengers, jelly-falls develop a white layer of bacteria over the decaying carcasses and emit a black residue over the surrounding area, which is from sulfide.[13] This high level of microbial activity requires a lot of oxygen, which can lead zones around jelly-falls to become hypoxic and inhospitable to larger scavengers.[13]
Research challenges
[edit]Researching jelly-falls relies on direct observational data such as video, photography, or benthic trawls.[1] A complication with trawling for jelly-falls is the gelatinous carcass easily falls apart and as a result, opportunistic photography, videography, and chemical analysis have been primary methods of monitoring.[3][9] This means that jelly-falls are not always observed in the time period in which they exist. Because jelly-falls can be fully processed and degraded within a number of hours by scavengers[9] and the fact that some jelly-falls will not sink below 500 m in tropical and subtropical waters,[5] the importance and prevalence of jelly-falls may be underestimated.
See also
[edit]References
[edit]- ^ a b c d e Lebrato, Mario; Pitt, Kylie A.; Sweetman, Andrew K.; Jones, Daniel O. B.; Cartes, Joan E.; Oschlies, Andreas; Condon, Robert H.; Molinero, Juan Carlos & Adler, Laetitia (2012). "Jelly-falls historic and recent observations: a review to drive future research directions". Hydrobiologia. 690 (1): 227–245. doi:10.1007/s10750-012-1046-8. S2CID 15428213.[permanent dead link]
- ^ Lebrato, M. & Jones, D. O. B. (2009). "Mass deposition event of Pyrosoma atlanticum carcasses off Ivory Coast (West Africa)" (PDF). Limnology and Oceanography. 54 (4): 1197–1209. Bibcode:2009LimOc..54.1197L. doi:10.4319/lo.2009.54.4.1197.
- ^ a b c d Sweetman, Andrew K. & Chapman, Annelise (2011). "First observations of jelly-falls at the seafloor in a deep-sea fjord". Deep Sea Research Part I: Oceanographic Research Papers. 58 (12): 1206–1211. Bibcode:2011DSRI...58.1206S. doi:10.1016/j.dsr.2011.08.006.
- ^ Burd, Adrian. "Towards a transformative understanding of the ocean's biological pump: Priorities for future research-Report on the NSF Biology of the Biological Pump Workshop" (PDF). OCB: Ocean Carbon & Biogeochemistry. Archived from the original (PDF) on 27 October 2016. Retrieved 30 October 2016.
- ^ a b c d e Lebrato, Mario; Pahlow, Markus; Oschlies, Andreas; Pitt, Kylie A.; Jones, Daniel O. B.; Molinero, Juan Carlos & Condon, Robert H. (2011). "Depth attenuation of organic matter export associated with jelly falls" (PDF). Limnology and Oceanography. 56 (5): 1917–1928. Bibcode:2011LimOc..56.1917L. doi:10.4319/lo.2011.56.5.1917. hdl:10072/43275. S2CID 3693276.
- ^ Purcell, J.E. (2012). "Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations". Annual Review of Marine Science. 4: 209–235. Bibcode:2012ARMS....4..209P. doi:10.1146/annurev-marine-120709-142751. PMID 22457974.
- ^ Lebrato, Mario; Molinero, Juan-Carlos; Cartes, Joan E.; Lloris, Domingo; Mélin, Frédéric & Beni-Casadella, Laia (2013). "Sinking jelly-carbon unveils potential environmental variability along a continental margin". PLOS ONE. 8 (12): e82070. Bibcode:2013PLoSO...882070L. doi:10.1371/journal.pone.0082070. PMC 3867349. PMID 24367499.
- ^ Lebrato, Mario; Pitt, Kylie A.; Sweetman, Andrew K.; Jones, Daniel O. B.; Cartes, Joan E.; Oschlies, Andreas; Condon, Robert H.; Molinero, Juan Carlos & Adler, Laetitia (2012). "Jelly-falls historic and recent observations: a review to drive future research directions". Hydrobiologia. 690 (1): 227–245. doi:10.1007/s10750-012-1046-8. S2CID 15428213.[permanent dead link]
- ^ a b c d Sweetman, Andrew K.; Smith, Craig R.; Dale, Trine & Jones, Daniel O. B. (2014). "Rapid scavenging of jellyfish carcasses reveals the importance of gelatinous material to deep-sea food webs". Proceedings of the Royal Society B: Biological Sciences. 281 (1796): 20142210. doi:10.1098/rspb.2014.2210. PMC 4213659. PMID 25320167.
- ^ Sweetman, Andrew & Chapman, Annaleise (2015). "First assessment of flux rates of jellyfish carcasses (jelly-falls) to the benthos reveals the importance of gelatinous material for biological C-cycling in jellyfish-dominated ecosystems". Frontiers in Marine Science. 2. doi:10.3389/fmars.2015.00047.
- ^ Tinta, Tinkara; Kogovšek, Tjaša; Turk, Valentina; Shiganova, Tamara A.; Mikaelyan, Alexander S. & Malej, Alenka (2016). "Microbial transformation of jellyfish organic matter affects the nitrogen cycle in the marine water column — A Black Sea case study". Journal of Experimental Marine Biology and Ecology. 475: 19–30. doi:10.1016/j.jembe.2015.10.018.
- ^ Sweetman, Andrew; Chelsky, Ariella; Pitt, Kylie Ann; Andrade, Hector; van Oevelen, Dick & Renaud, Paul (2016). "Jellyfish decomposition at the seafloor rapidly alters biogeochemical cycling and carbon flow through benthic food-websJellyfish decomposition at the seafloor rapidly alters biogeochemical cycling and carbon flow through benthic food-webs". Limnology and Oceanography. 61 (4): 1449–1461. Bibcode:2016LimOc..61.1449S. doi:10.1002/lno.10310. hdl:10072/142821.
- ^ a b West, Elizabeth Jane; Welsh, David Thomas & Pitt, Kylie Anne (2009). "Influence of decomposing jellyfish on the sediment oxygen demand and nutrient dynamics". Hydrobiologia. 616 (1): 151–160. doi:10.1007/s10750-008-9586-7. S2CID 46695384.
