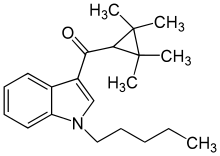
UR-144
 | |
| Clinical data | |
|---|---|
| Trade names | different brandnames, typical example: "TMCP-018", "KM-X1", "UR-144", "MN-001", "YX-17" |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H29NO |
| Molar mass | 311.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories,[2] that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor.
Pharmacology
[edit]UR-144 has high affinity for the CB2 receptor with a Ki of 1.8 nM but 83x lower affinity for the CB1 receptor with a Ki of 150 nM.[3] UR-144 was found to possess an EC50 of 421 nM for human CB1 receptors, and 72 nM for human CB2 receptors.[4] UR-144 produces bradycardia and hypothermia in rats at a dose of 10 mg/kg, suggesting weak cannabinoid-like activity.[4]
Chemically it is closely related to other 2,2,3,3-tetramethylcyclopropyl synthetic cannabinoids like A-796,260 and A-834,735 but with a different substitution on the 1-position of the indole core, in these compounds its 1-pentyl group is replaced with alkylheterocycles like 1-(2-morpholinoethyl) and 1-(tetrahydropyran-4-ylmethyl).
Legality
[edit]The UK ACMD recommended that generic prohibition legislation be extended to include UR-144 in October 2012.[5] The UK Home Office accepted the recommendation and enacted legislation to ban UR-144 as a class B drug along with a number of other drugs on February 26, 2013 as a part of The Misuse of Drugs Act 1971 (Amendment) Order 2013.
UR-144 was detected in Korea, 2012. This molecule is very close to KM-X1, MN-001, YX-17 and Kr-11.[6]
UR-144 (Abbott patent) has been detected as an ingredient of synthetic cannabis smoking blends in New Zealand, and subsequently banned from sale as a temporary class drug on 6 April 2012.[7] It has also been encountered in smoking blends and subsequently banned in Russia.[8]
As of October 2015 UR-144 is a controlled substance in China.[9]
UR-144 is banned in the Czech Republic.[10]
Detection
[edit]A forensic standard of UR-144 is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[11] An ELISA immunoassay technique for detecting UR-144 in urine as part of general drug screens has been developed by Tulip Biolabs, Inc. An Homogeneous Immunoassay that runs on most Clinical Chemistry Analyzers and detects several UR and XLR synthetic cannabinoids has been developed and introduced by Immunalysis Inc. Pomona USA.
See also
[edit]- AB-001
- AM-1221
- 4-HTMPIPO
- FAB-144
- JTE 7-31
- JWH-018
- N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide
- XLR-11
- Tetramethylcyclopropylfentanyl
References
[edit]- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ WO application 2006069196, Pace JM, Tietje K, Dart MJ, Meyer MD, "3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands", published 2006-06-29, assigned to Abbott Laboratories
- ^ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- ^ a b Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, et al. (August 2015). "Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience. 6 (8): 1445–1458. doi:10.1021/acschemneuro.5b00107. PMID 25921407.
- ^ "Further consideration of the synthetic cannabinoids". UK Advisory Council on the Misuse of Drugs. 18 October 2012. p. 14. Retrieved 13 December 2014.
- ^ Almada M, Alves P, Fonseca BM, Carvalho F, Queirós CR, Gaspar H, et al. (February 2020). "Synthetic cannabinoids JWH-018, JWH-122, UR-144 and the phytocannabinoid THC activate apoptosis in placental cells". Toxicology Letters. 319: 129–137. doi:10.1016/j.toxlet.2019.11.004. PMID 31730886. S2CID 208061836.
- ^ Temporary Class Drug Notices. New Zealand Ministry of Health
- ^ Sobolevsky T, Prasolov I, Rodchenkov G (October 2012). "Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids". Drug Testing and Analysis. 4 (10): 745–753. doi:10.1002/dta.1418. PMID 23042760.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- ^ "Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)" (PDF) (in Czech). Ministerstvo zdravotnictví.
- ^ Southern Association of Forensic Scientists http://forendex.southernforensic.org/index.php/detail/index/1218
Further reading
[edit]- Poso A, Huffman JW (January 2008). "Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands". British Journal of Pharmacology. 153 (2): 335–346. doi:10.1038/sj.bjp.0707567. PMC 2219524. PMID 17982473.
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, et al. (January 2008). "Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI". British Journal of Pharmacology. 153 (2): 367–379. doi:10.1038/sj.bjp.0707506. PMC 2219521. PMID 17965748.
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (March 2008). "Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity". Journal of Medicinal Chemistry. 51 (6): 1904–1912. doi:10.1021/jm7011613. PMID 18311894.
