Transition metal pyridine complexes

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.
Bonding
[edit]With a pKa of 5.25 for its conjugate acid, pyridine is about 15x less basic than imidazole. Pyridine is a weak pi-acceptor ligand. Trends in the M-N distances for complexes of the type [MCl2(py)4]2+ reveal an anticorrelation with d-electron count.[2] Few low-valent metal complexes of pyridines are known. The role of pyridine as a Lewis base extends also to main group chemistry. Examples include sulfur trioxide pyridine complex SO3(py) and pyridine adduct of borane, BH3py.
Classification of metal-pyridine complexes
[edit]Many metal pyridine complexes are known. These complexes can be classified according to their geometry, i.e. octahedral, tetrahedral, linear, etc.
Octahedral complexes
[edit]

Owing to the relatively wide C-N-C angle, the 2,6-hydrogen atoms interfere with the formation of [M(py)6]z complexes. A few octahedral homoleptic pyridine complexes are known. These complex cations are found in the salts [Ru(py)6]Fe4(CO)13 and [Ru(py)6](BF4)2.[3][4] Some compounds with the stoichiometry M(py)6(ClO4)2 have been reformulated as [M(py)4(ClO4)2].(py)2 [5]
A common family of pyridine complexes are of the type [MCl2(py)4]n+. The chloride ligands are mutually trans in these complexes.
| formula | CAS RN | key properties | Preparation | |
|---|---|---|---|---|
| TiCl2(pyridine)4 | 131618-68-3 | blue, triplet dTi-N=2.27 Å, dTi-Cl = 2.50 Å (thf solvate)[6] |
TiCl3(thf)3 + KC8 + py[7] | |
| VCl2(pyridine)4 | 15225-42-0 | purple[8] | VCl3 + Zn + py[9] | |
| CrCl2(pyridine)4 | 51266-53-6 | green dCr-Cl = 2.80 Å dCo-Cl = 2.16 Å |
CrCl2 + py[10] | |
| MnCl2(pyridine)4 | 14638-48-3 | 1.383 | ||
| FeCl2(pyridine)4 | 15138-92-8 | yellow dFe-Cl = 2.43 Å |
FeCl2 + py[2] | |
| CoCl2(pyridine)4 | 13985-87-0 | blue dCo-Cl = 2.44 Å |
CoCl2 + py[2] | |
| [CoCl2(pyridine)4]Cl | 27883-34-7 | green (hexahydrate) dCo-Cl = 2.25 Å, dCo-N = 1.98 Å[11] as [CoCl3(py)]− salt |
CoCl2(pyridine)4 + Cl2[12] | |
| NiCl2(pyridine)4 | 14076-99-4 | blue dNi-Cl = 2.44 Å |
NiCl2 + py[2] | |
| NbCl2(pyridine)4 | 168701-43-7 | dNb-N = 2.22 Å, dNb-Cl = 2.51 Å | NbCl4(thf)2 + KC8 + py[6] | |
| [MoCl2py)4]Br3 | Br3− salt[13] | yellow dMo-Cl= 2.41 Å, dMo-N=2.20 Å |
||
| TcCl2py)4 | 172140-87-3 | purple dTc-Cl = 2.41 Å, dTc-N = 2.10 Å[14] |
TcCl4py2 + Zn + py | |
| RuCl2(pyridine)4 | 16997-43-6 | red-orange dRu-N=2.08 Å, dRu-Cl=2.40 Å |
RuCl3(H2O)x + py [15] | |
| [RhCl2(pyridine)4]+ | 14077-30-6 (Cl− salt) | yellow | RhCl3(H2O)3 + py + cat. reductant[16] | |
| OsCl2(pyridine)4 | 137822-02-7 | brown dOs-Cl = 2.40 Å, dOs-N= 2.068 Å |
K3OsCl6 + py + (CH2OH)2/140 °C[17] | |
| [IrCl2(pyridine)4]+ | yellow 1.35 Å (chloride.hexahydrate)[18] |
The tris(pyridine) trihalides, i.e., [MCl3(py)3] (M = Ti, Cr, Rh[19] Ir), are another large class of M-Cl-py complexes.
Four-coordinate complexes
[edit]
Four-coordinate complexes include tetrahedral and square planar derivatives. Examples of homoleptic tetrahedral complexes include [M(py)4]n+ for Mn+ = Cu+,[21] M = Ni2+,[22] Ag+,[23] and Ag2+.[24] Examples of homoleptic square planar complexes include the d8 cations [M(py)4]n+ for Mn+ = Pd2+,[25] Pt2+,[26] Au3+.[27]
Ni(ClO4)2(3-picoline)2 can be isolated in two isomers, yellow, diamagnetic square planar or blue, paramagnetic tetrahedral.[28]
Mn(II) and Co(II) form both tetrahedral MCl2py2 and octahedral MCl2py4 complexes, depending on conditions:[29]
- MCl2py2 + 2 py → MCl2py4
Two- and three-coordinate complexes
[edit]Many examples exist for [Au(py)2]+.[27] [Ag(py)3]+ and [Cu(py)2]+ are also precedented.[30][27]
Pi-complexes
[edit]The η6 coordination mode, as occurs in η6 benzene complexes, is observed only in sterically encumbered derivatives that block the nitrogen center.[31]
Comparison with related ligands
[edit]- Picolines
Many substituted pyridines function as ligands for transition metals. The monomethyl derivatives, the picolines (2-, 3-, and 4-picoline), are best studied. 2-Picolines are sterically impeded from coordination.[28]
- 2,2'-bipy
Coupling of two pyridine rings at their 2-positions gives 2,2'-bipyridine, a widely studied bidentate ligand. A number of differences are apparent between pyridine and bipyridine complexes. Many [M(bipy)3]z complexes are known, whereas analogous [M(py)6]z complexes are rare and apparently labile. Bipyridine is a redox-noninnocent ligand, as illustrated by the existence of complexes such as [Cr(bipy)3]0. The pyridine analogues of such complexes are unknown. The dichloro complexes [MCl2(bipy)2]n+ tend to be cis, as exemplified by RuCl2(bipy)2. In contrast, the complexes [MCl2(py)4]n+ are always trans.
- Imidazoles
Imidazoles comprise another major series of N-heterocyclic ligands. Unlike pyridines, imidazole derivatives are common ligands in nature.
Applications and occurrence
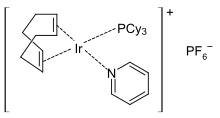
[edit]Crabtree's catalyst, a popular catalyst for hydrogenations, is a pyridine complex.
Although transition metal pyridine complexes have few practical applications, they are widely used synthetic precursors. Many are anhydrous, soluble in nonpolar solvents, and susceptible to alkylation by organolithium and Grignard reagents. Thus CoCl2(py)4 has proven very useful in organocobalt chemistry[32][33] and NiCl2(py)4 useful in organonickel chemistry.[34]
References
[edit]- ^ Shin, Yeung-gyo K.; Szalda, David J.; Brunschwig, Bruce S.; Creutz, Carol; Sutin, Norman (1997). "Electronic and Molecular Structures of Pentaammineruthenium Pyridine and Benzonitrile Complexes as a Function of Oxidation State". Inorganic Chemistry. 36 (14): 3190–3197. doi:10.1021/ic9700967. PMID 11669976.
- ^ a b c d Long, Gary J.; Clarke, Peter J. (1978). "Crystal and Molecular Structures of trans-Tetrakis(pyridine)dichloroiron(II), -Nickel(II), and -Cobalt(II) and trans-Tetrakis(pyridine)dichloroiron(II) Monohydrate". Inorganic Chemistry. 17 (6): 1394–1401. doi:10.1021/ic50184a002.
- ^ Templeton, Joseph L. (1979). "Hexakis(pyridine)ruthenium(II) tetrafluoroborate. Molecular structure and spectroscopic properties". Journal of the American Chemical Society. 101 (17): 4906–4917. doi:10.1021/ja00511a020.
- ^ Lichtenberg, Crispin; Adelhardt, Mario; Wörle, Michael; Büttner, Torsten; Meyer, Karsten; Grützmacher, Hansjörg (2015). "Mono- and Dinuclear Neutral and Cationic Iron(II) Compounds Supported by an Amidinato-diolefin Ligand: Characterization and Catalytic Application". Organometallics. 34 (12): 3079–3089. doi:10.1021/acs.organomet.5b00395.
- ^ Soldatov, Dmitriy V.; Ripmeester, John A. (1998). "Hexapyridine Addition Compounds of Metal(II) Salts (Nickel and Magnesium Perchlorates, Magnesium Nitrate): Their Supramolecular Nature, and Anion Coordination of the Metal Cation [1]". Supramolecular Chemistry. 9 (3): 175–181. doi:10.1080/10610279808034984.
- ^ a b Araya, Miguel A.; Cotton, F. Albert; Matonic, John H.; Murillo, Carlos A. (1995). "An Efficient Reduction Process Leading to Titanium(II) and Niobium(II): Preparation and Structural Characterization of trans-MCl2(py)4 Compounds, M = Ti, Nb, and Mn". Inorganic Chemistry. 34 (22): 5424–5428. doi:10.1021/ic00126a009.
- ^ Wijeratne, Gayan B.; Zolnhofer, Eva M.; Fortier, Skye; Grant, Lauren N.; Carroll, Patrick J.; Chen, Chun-Hsing; Meyer, Karsten; Krzystek, J.; Ozarowski, Andrew; Jackson, Timothy A.; Mindiola, Daniel J.; Telser, Joshua (2015). "Electronic Structure and Reactivity of a Well-Defined Mononuclear Complex of Ti(II)". Inorganic Chemistry. 54 (21): 10380–10397. doi:10.1021/acs.inorgchem.5b01796. PMID 26451744.
- ^ Brauer, D. J.; Krüger, C. (1973). "Tetrapyridinedichlorovanadium(II)". Cryst. Struct. Commun. 2: 421.
- ^ Edema, Jilles J. H.; Stauthamer, Walter; Van Bolhuis, Fre; Gambarotta, Sandro; Smeets, Wilberth J. J.; Spek, Anthony L. (1990). "Novel Vanadium(II) Amine Complexes: A Facile Entry in the chemistry of Divalent Vanadium. Synthesis and Characterization of Mononuclear L4VCl2 [L = Amine, Pyridine]: X-ray Structures of trans-(TMEDA)2VCl2 [TMEDA = N,N,N',N'-Tetramethylethylenediamine] and trans-Mz2V(py)2 [Mz = o-C6H4CH2N(CH3)2, py = Pyridine]". Inorganic Chemistry. 29 (7): 1302–1306. doi:10.1021/ic00332a003.
- ^ Cotton, F. Albert; Daniels, Lee M.; Feng, Xuejun; Maloney, David J.; Murillo, Carlos A.; Zúñiga, Luis A. (1995). "Experimental and Theoretical study of a Paradigm Jahn-Teller Molecule, all-trans-CrCl2(H2O)2(pyridine)2, and the Related trans-CrCl2(pyridine)4·acetone". Inorganica Chimica Acta. 235 (1–2): 21–28. doi:10.1016/0020-1693(95)90041-4.
- ^ Xu, Hao; Li, Jinyu; Wu, Zhongzhi; Zou, Jianzhong; Xu, Zheng; You, Xiaozeng; Dong, Zhengchao (1993). "Synthesis and X-ray Crystal Structure of a Mixed-Valent Cobalt Complex, Co2Cl5(py)5". Polyhedron. 12 (18): 2261–2264. doi:10.1016/S0277-5387(00)88265-0.
- ^ Glerup, Jørgen; Schäffer, Claus Erik; Springborg, Johan (1978). "Synthesis of trans-Dichloro- and trans-Difluorotetrakis(pyridine)cobalt(III) Salts". Acta Chemica Scandinavica. 32a: 673–674. doi:10.3891/acta.chem.scand.32a-0673.
- ^ Rotar, R.; Leban, I.; Brenčič, J. V. (1996). "Trans-Dichlorotetrakis(pyridine-N)molybdenum(III) Tribromide". Acta Crystallographica Section C Crystal Structure Communications. 52 (9): 2155–2157. doi:10.1107/S0108270196005628.
- ^ Barrera, Joseph; Burrell, Anthony K.; Bryan, Jeffrey C. (1996). "Technetium(III), Technetium(II), and Technetium(I) Complexes with Pyridine Ligands. Can Pyridine Coordination Stabilize the Low Oxidation States of Technetium?". Inorganic Chemistry. 35 (2): 335–341. doi:10.1021/ic950291q. PMID 11666213.
- ^ Wong, W. T.; Lau, T. C. (1994). "trans-Dichlorotetrapyridineruthenium(II)". Acta Crystallographica Section C Crystal Structure Communications. 50 (9): 1406–1407. doi:10.1107/S0108270194002088. hdl:10722/69086.
- ^ Gillard, R. D.; Wilkinson, G. W. (1967). "Trans -Dichlorotetra(pyridine)Rhodium(III) Salts". trans-Dichlorotetra(pyridine)rhodium(III) Salts. Inorganic Syntheses. Vol. 10. pp. 64–67. doi:10.1002/9780470132418.ch11. ISBN 9780470132418.
- ^ Kolf, S.; Preetz, W. (1997). "Darstellung, Kristallstrukturen, Schwingungsspektren und Normalkoordinatenanalysen der trans-Dihalogeno-tetrakis-Pyridin-Osmium(II)-Komplexe trans-[OsX2Py4], X = F, Cl, Br, I". Zeitschrift für anorganische und allgemeine Chemie. 623 (1–6): 501–508. doi:10.1002/zaac.19976230179.
- ^ Gillard, R. D.; Mitchell, Simon H.; Williams, Peter A.; Vagg, Robert S. (1984). "The Structure of a Low-Temperature Form of trans-[Ir(pyridine)4Cl2]Cl. 6H2O". Journal of Coordination Chemistry. 13 (4): 325–330. doi:10.1080/00958978408073886.
- ^ Acharya, K. R.; Tavale, S. S.; Guru Row, T. N. (1984). "Structure of mer-Trichlorotris(pyridine)rhodium(III), [RhCl3(C5H5N)3]". Acta Crystallographica Section C Crystal Structure Communications. 40 (8): 1327–1328. doi:10.1107/S0108270184007848.
- ^ J. C. Collins, W.W. Hess (1972). "Aldehydes from Primary Alcohols by Oxidation with Chromium Trioxide: Heptanal". Organic Syntheses. 52: 5. doi:10.15227/orgsyn.052.0005.
- ^ Horvat, Gordan; Portada, Tomislav; Stilinović, Vladimir; Tomišić, Vladislav (2007). "Tetrapyridinecopper(I) hexafluoridophosphate(V)". Acta Crystallographica Section E. 63 (6): m1734. doi:10.1107/S1600536807024051.
- ^ Liptay, G.; Wadsten, T.; Borbély-Kuszmann, A. (1986). "Characterization of [Ni(py)4]Cl2 and its thermal decomposition". Journal of Thermal Analysis. 31 (4): 845–852. doi:10.1007/BF01913555. S2CID 93538201.
- ^ Nilsson, Karin; Oskarsson, Åke; Lund, P.-A.; Shen, Quang; Weidlein, Johan; Spiridonov, V. P.; Strand, T. G. (1982). "The Crystal Structure of Tetrapyridine Copper(I) Perchlorate and Tetrapyridine Silver(I) Perchlorate at 260 K". Acta Chemica Scandinavica. 36a: 605–610. doi:10.3891/acta.chem.scand.36a-0605.
- ^ Kauffman, George B.; Houghten, Richard A.; Likins, Robert E.; Posson, Philip L.; Ray, R. K. (2007) [1998]. "Tetrakis(Pyridine)Silver(2+)Peroxydisulfate". Inorganic Syntheses. Inorganic Syntheses. Vol. 32. pp. 177–181. doi:10.1002/9780470132630.ch30. ISBN 9780470132630.
- ^ Corbo, Robert; Georgiou, Dayne C.; Wilson, David J. D.; Dutton, Jason L. (2014). "Reactions of [PhI(pyridine)2]2+ with Model Pd and Pt II/IV Redox Couples". Inorganic Chemistry. 53 (3): 1690–1698. doi:10.1021/ic402836d. PMID 24409820.
- ^ Wei, C. H.; Hingerty, B. E.; Busing, W. R. (1989). "Structure of Tetrakis(pyridine)platinum(II) chloride trihydrate: Unconstrained anisotropic least-squares refinement of hydrogen and non-hydrogen atoms from combined X-ray–neutron diffraction data". Acta Crystallographica Section C Crystal Structure Communications. 45: 26–30. doi:10.1107/S0108270188009515.
- ^ a b c Corbo, Robert; Ryan, Gemma F.; Haghighatbin, Mohammad A.; Hogan, Conor F.; Wilson, David J. D.; Hulett, Mark D.; Barnard, Peter J.; Dutton, Jason L. (2016). "Access to the Parent Tetrakis(pyridine)gold(III) Trication, Facile Formation of Rare Au(III) Terminal Hydroxides, and Preliminary Studies of Biological Properties". Inorganic Chemistry. 55 (6): 2830–2839. doi:10.1021/acs.inorgchem.5b02667. PMID 26930516.
- ^ a b Vallarino, L. M.; Hill, W. E.; Quagliano, J. V. (1965). "Coordination Compounds of Nickel(II) Salts with Substituted Pyridines. Complexes of 2-, 3-, and 4-Methylpyridine". Inorganic Chemistry. 4 (11): 1598–1604. doi:10.1021/ic50033a014.
- ^ Libug, W.; Uruska, I. (1966). "The Relative Stability of Octahedral and Tetrahedral Complexes in Solution. I. Chloropyridine Complexes of Divalent Transition Metals". Inorganic Chemistry. 5 (2): 256–264. doi:10.1021/ic50036a022.
- ^ Yang, Wenbin; Lu, Canzhong; Zhuang, Honghui (2002). "Hydrothermal Synthesis and Structures of Three New Copper Complexes: [{Cu(2,2′-bipy}2(β-Mo8O26)], [{Cu(py)3}2{Cu(py)2}2(α-Mo8O26)] and [Cu(py)2]4[(SO4)Mo12O36]". Journal of the Chemical Society, Dalton Transactions (14): 2879–2884. doi:10.1039/b111480h.
- ^ Elschenbroich, C. (2008). Organometallchemie (6th ed.). Vieweg & Teubner. pp. 524–525. ISBN 978-3-8351-0167-8.
- ^ Zhu, Di; Janssen, Femke F. B. J.; Budzelaar, Peter H. M. (2010). "(Py)2Co(CH2SiMe3)2 as an Easily Accessible Source of "CoR2"". Organometallics. 29 (8): 1897–1908. doi:10.1021/om901045s.
- ^ Chirik, Paul J. (2015). "Iron- and Cobalt-Catalyzed Alkene Hydrogenation: Catalysis with Both Redox-Active and Strong Field Ligands". Accounts of Chemical Research. 48 (6): 1687–1695. doi:10.1021/acs.accounts.5b00134. PMID 26042837.
- ^ Cámpora, Juan; Del Mar Conejo, Marı́a; Mereiter, Kurt; Palma, Pilar; Pérez, Carmen; Reyes, Manuel L.; Ruiz, Caridad (2003). "Synthesis of Dialkyl, Diaryl and Metallacyclic Complexes of Ni and Pd Containing Pyridine, α-Diimines and Other Nitrogen Ligands". Journal of Organometallic Chemistry. 683: 220–239. doi:10.1016/S0022-328X(03)00691-0.
