Microaerophile
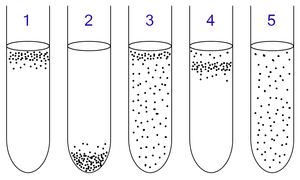
1: Obligate aerobes need oxygen because they cannot ferment or respire anaerobically. They gather at the top of the tube where the oxygen concentration is highest.
2: Obligate anaerobes are poisoned by oxygen, so they gather at the bottom of the tube where the oxygen concentration is lowest.
3: Facultative anaerobes can grow with or without oxygen because they can metabolise energy aerobically or anaerobically. They gather mostly at the top because aerobic respiration generates more ATP than either fermentation or anaerobic respiration.
4: Microaerophiles need oxygen because they cannot ferment or respire anaerobically. However, they are poisoned by high concentrations of oxygen. They gather in the upper part of the test tube but not the very top.
5: Aerotolerant organisms do not require oxygen as they metabolise energy anaerobically. Unlike obligate anaerobes however, they are not poisoned by oxygen. They can be found evenly spread throughout the test tube.
A microaerophile is a microorganism that requires environments containing lower levels of dioxygen than that are present in the atmosphere (i.e. < 21% O2; typically 2–10% O2) for optimal growth.[1] A more restrictive interpretation requires the microorganism to be obligate in this requirement.[2][3] Many microaerophiles are also capnophiles, requiring an elevated concentration of carbon dioxide (e.g. 10% CO2 in the case of Campylobacter species).[4]
The original definition of a microaerophile has been criticized for being too restrictive and not accurate enough compared to similar categories. The broader term microaerobe has been coined to describe microbes able to respire oxygen "within microoxic environments by using high-affinity terminal oxidase".[1]
Culture
[edit]Microaerophiles are traditionally cultivated in candle jars. Candle jars are containers into which a lit candle is introduced before sealing the container's airtight lid. The candle's flame burns until extinguished by oxygen deprivation, creating a carbon dioxide-rich, oxygen-poor atmosphere.[5]
Newer oxystat bioreactor methods allow for more precise control of gas levels in the microaerobic environment, using a probe to measure the oxygen concentration or redox potential in real time.[6] Ways to control oxygen intake include gas-generating packs and gas exchange.[4]
As oxystat bioreactors are expensive to buy and run, lower-cost solutions have been devised. For example, the Micro-Oxygenated Culture Device (MOCD) is a system involving ordinary flasks, oxygen-permeable tubes, sensors, and water pumps. Aeration is done by pumping the culture medium through the tubes.[1]
Examples
[edit]A wide variety of microaerobic conditions exist in the world: in human bodies, underwater, etc. Many bacteria from these sources are microaerobes, some of which are also microaerophiles.
- Some members of Campylobacterales are microaerophilic:
- Campylobacter species are microaerophilic.[4][7]
- Helicobacter pylori (previously identified as a Campylobacter), a species of Campylobacterota that has been linked to peptic ulcers and some types of gastritis[8]
- Many members of Lactobacillus sensu lato (see Lactobacillaceae) are microaerophiles. As facultative anaerobes, they do survive anaerobic conditions, but grow better with a little oxygen.[9]
- Magnetospirillum gryphiswaldense and Magnetospira sp. QH-2 are aquatic microaerophilic magnetotactic bacteria. The formation of magnetite in such bacteria in general require microaerobic conditions.[1]
See also
[edit]- Aerobic respiration
- Anaerobic respiration
- Facultative anaerobe
- Fermentation
- Obligate aerobe
- Obligate anaerobe
- Oxygenation (environmental)
References
[edit]- ^ a b c d Fuduche, Maxime; Davidson, Sylvain; Boileau, Céline; Wu, Long-Fei; Combet-Blanc, Yannick (19 March 2019). "A Novel Highly Efficient Device for Growing Micro-Aerophilic Microorganisms". Frontiers in Microbiology. 10: 534. doi:10.3389/fmicb.2019.00534. PMC 6434946. PMID 31001208.
- ^ Hogg, S. (2005). Essential Microbiology (1st ed.). Wiley. pp. 91–107. ISBN 0-471-49754-1.
- ^ Prescott LM, Harley JP, Klein DA (1996). Microbiology (3rd ed.). Wm. C. Brown Publishers. pp. 130–131. ISBN 0-697-29390-4.
- ^ a b c Brooks GF, Carroll KC, Butel JS, Morse SA (2007). Jawetz, Melnick & Adelberg's Medical Microbiology (24th ed.). McGraw Hill. pp. 273–275. ISBN 978-0-07-128735-7.
- ^ Salim SM, Mandal J, Parija SC (March 2014). "Isolation of Campylobacter from human stool samples". Indian J Med Microbiol. 32 (1): 35–38. doi:10.4103/0255-0857.124294. PMID 24399385.
- ^ Liu, Chen-Guang; Xue, Chuang; Lin, Yen-Han; Bai, Feng-Wu (March 2013). "Redox potential control and applications in microaerobic and anaerobic fermentations". Biotechnology Advances. 31 (2): 257–265. doi:10.1016/j.biotechadv.2012.11.005. PMID 23178703.
- ^ Fernie DS, Park RW (August 1977). "The isolation and nature of campylobacters (microaerophilic vibrios) from laboratory and wild rodents". J. Med. Microbiol. 10 (3): 325–9. doi:10.1099/00222615-10-3-325. PMID 330861.
- ^ Cover TL (2012). "Perspectives on Methodology for in Vitro Culture of Helicobacter pylori". Perspectives on methodology for in vitro culture of Helicobacter pylori. Methods Mol Biol. Vol. 921. pp. 11–15. doi:10.1007/978-1-62703-005-2_3. ISBN 978-1-62703-004-5. PMC 3921885. PMID 23015486.
- ^ Goldstein, Ellie J. C.; Tyrrell, Kerin L.; Citron, Diane M. (15 May 2015). "Lactobacillus Species: Taxonomic Complexity and Controversial Susceptibilities". Clinical Infectious Diseases. 60 (suppl_2): S98–S107. doi:10.1093/cid/civ072. PMID 25922408.
External links
[edit]
