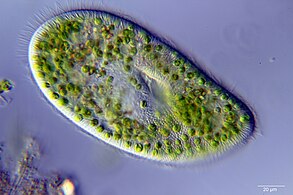
Mixotroph
A mixotroph is an organism that uses a mix of different sources of energy and carbon, instead of having a single trophic mode, on the continuum from complete autotrophy to complete heterotrophy. It is estimated that mixotrophs comprise more than half of all microscopic plankton.[1] There are two types of eukaryotic mixotrophs. There are those with their own chloroplasts - including those with endosymbionts providing the chloroplasts. And there are those that acquire them through kleptoplasty, or through symbiotic associations with prey, or through 'enslavement' of the prey's organelles.[2]
Possible combinations are photo- and chemotrophy, litho- and organotrophy (osmotrophy, phagotrophy and myzocytosis), auto- and heterotrophy or other combinations of these. Mixotrophs can be either eukaryotic or prokaryotic.[3] They can take advantage of different environmental conditions.[4]
If a trophic mode is obligate, then it is always necessary for sustaining growth and maintenance; if facultative, it can be used as a supplemental source.[3] Some organisms have incomplete Calvin cycles, so they are incapable of fixing carbon dioxide and must use organic carbon sources.
Overview
[edit]Organisms may employ mixotrophy obligately or facultatively.
- Obligate mixotrophy: To support growth and maintenance, an organism must utilize both heterotrophic and autotrophic means.
- Obligate autotrophy with facultative heterotrophy: Autotrophy alone is sufficient for growth and maintenance, but heterotrophy may be used as a supplementary strategy when autotrophic energy is not enough, for example, when light intensity is low.
- Facultative autotrophy with obligate heterotrophy: Heterotrophy is sufficient for growth and maintenance, but autotrophy may be used to supplement, for example, when prey availability is very low.
- Facultative mixotrophy: Maintenance and growth may be obtained by heterotrophic or autotrophic means alone, and mixotrophy is used only when necessary.[5]
Plants
[edit]
Amongst plants, mixotrophy classically applies to carnivorous, hemi-parasitic and myco-heterotrophic species. However, this characterisation as mixotrophic could be extended to a higher number of clades as research demonstrates that organic forms of nitrogen and phosphorus—such as DNA, proteins, amino-acids or carbohydrates—are also part of the nutrient supplies of a number of plant species.[6]
Animals
[edit]Mixotrophy is less common among animals than among plants and microbes, but there are many examples of mixotrophic invertebrates and at least one example of a mixotrophic vertebrate.
- The spotted salamander, Ambystoma maculatum, also hosts microalgae within its cells. Its embryos have been found to have symbiotic algae living inside them,[7] the only known example of vertebrate cells hosting an endosymbiont microbe (unless mitochondria is considered).[8][9]
- Zoochlorella is a nomen rejiciendum for a genus of green algae assigned to Chlorella.[10] The term zoochlorella (plural zoochlorellae) is sometimes used to refer to any green algae that lives symbiotically within the body of a freshwater or marine invertebrate or protozoan.
- Reef-building corals (Scleractinia), like many other cnidarians (e.g. jellyfish, anemones), host endosymbiotic microalgae within their cells, thus making them mixotrophs.
- The Oriental hornet, Vespa orientalis, can obtain energy from sunlight absorbed by its cuticle.[11] It thus contrasts with the other animals listed here, which are mixotrophic with the help of endosymbionts.
-
Zooxanthellae is a photosynthetic algae that lives inside hosts like coral.
-
Anthopleura xanthogrammica gains its green colour from Zoochlorella.
-
The spotted jelly, a mixotrophic jellyfish, lives in trophic mutualism with zooxanthella, a unicellular organism capable of photosynthesis.[12]
Microorganisms
[edit]Bacteria and archaea
[edit]- Paracoccus pantotrophus is a bacterium that can live chemoorganoheterotrophically, whereby a large variety of organic compounds can be metabolized. Also a facultative chemolithoautotrophic metabolism is possible, as seen in colorless sulfur bacteria (some Thiobacillus), whereby sulfur compounds such as hydrogen sulfide, elemental sulfur, or thiosulfate are oxidized to sulfate. The sulfur compounds serve as electron donors and are consumed to produce ATP. The carbon source for these organisms can be carbon dioxide (autotrophy) or organic carbon (heterotrophy).[13][14][15]
Organoheterotrophy can occur under aerobic or under anaerobic conditions; lithoautotrophy takes place aerobically.[16][17]
Protists
[edit]
DIN = dissolved inorganic nutrients
To characterize the sub-domains within mixotrophy, several very similar categorization schemes have been suggested. Consider the example of a marine protist with heterotrophic and photosynthetic capabilities: In the breakdown put forward by Jones,[19] there are four mixotrophic groups based on relative roles of phagotrophy and phototrophy.
- A: Heterotrophy (phagotrophy) is the norm, and phototrophy is only used when prey concentrations are limiting.
- B: Phototrophy is the dominant strategy, and phagotrophy is employed as a supplement when light is limiting.
- C: Phototrophy results in substances for both growth and ingestion, phagotrophy is employed when light is limiting.
- D: Phototrophy is most common nutrition type, phagotrophy only used during prolonged dark periods, when light is extremely limiting.
An alternative scheme by Stoeker[18] also takes into account the role of nutrients and growth factors, and includes mixotrophs that have a photosynthetic symbiont or who retain chloroplasts from their prey. This scheme characterizes mixotrophs by their efficiency.
- Type 1: "Ideal mixotrophs" that use prey and sunlight equally well
- Type 2: Supplement phototrophic activity with food consumption
- Type 3: Primarily heterotrophic, use phototrophic activity during times of very low prey abundance.[21]
Another scheme, proposed by Mitra et al., specifically classifies marine planktonic mixotrophs so that mixotrophy can be included in ecosystem modeling.[20] This scheme classified organisms as:
- Constitutive mixtotrophs (CMs): phagotrophic organisms that are inherently able to also photosynthesize
- Non-constitutive mixotrophs (NCMs): phagotrophic organisms that must ingest prey to attain the ability to photosynthesize. NCMs are further broken down into:
- Specific non-constitutive mixotrophs (SNCMs), which only gain the ability to photosynthesize from a specific prey item (either by retaining plastids only in kleptoplastidy or by retaining whole prey cells in endosymbiosis)
- General non-constitutive mixotrophs (GNCM), which can gain the ability to photosynthesize from a variety of prey items
(A) phagotrophic (no phototrophy); (B) phototrophic (no phagotrophy); (C) constitutive mixotroph, with innate capacity for phototrophy; (D) generalist non-constitutive mixotroph acquiring photosystems from different phototrophic prey; (E) specialist non-constitutive mixotroph acquiring plastids from a specific prey type; (F) specialist non-constitutive mixotroph acquiring photosystems from endosymbionts. DIM = dissolved inorganic material (ammonium, phosphate etc.). DOM = dissolved organic material
-
Acantharian radiolarian hosts Phaeocystis symbionts.
-
White Phaeocystis algal foam washing up on a beach
-
Euglena mutabilis, a photosynthetic flagellate
-
Fluorescent micrograph of an acantharian with Phaeocystis symbionts fluorescing red (chlorophyll)
See also
[edit]Notes
[edit]- ^ Beware the mixotrophs - they can destroy entire ecosystems 'in a matter of hours'
- ^ [S. G. Leles et al, Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance, Proceedings of the Royal Society B: Biological Sciences (2017).]
- ^ a b Eiler A (December 2006). "Evidence for the Ubiquity of Mixotrophic Bacteria in the Upper Ocean: Implications and Consequences". Appl Environ Microbiol. 72 (12): 7431–7. Bibcode:2006ApEnM..72.7431E. doi:10.1128/AEM.01559-06. PMC 1694265. PMID 17028233.
- ^ Katechakis A, Stibor H (July 2006). "The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions". Oecologia. 148 (4): 692–701. Bibcode:2006Oecol.148..692K. doi:10.1007/s00442-006-0413-4. PMID 16568278. S2CID 22837754.
- ^ Schoonhoven, Erwin (January 19, 2000). "Ecophysiology of Mixotrophs" (PDF). Thesis.
- ^ Schmidt, Susanne; John A. Raven; Chanyarat Paungfoo-Lonhienne (2013). "The mixotrophic nature of photosynthetic plants". Functional Plant Biology. 40 (5): 425–438. doi:10.1071/FP13061. ISSN 1445-4408. PMID 32481119.
- ^ Petherick, Anna (2010-07-30). "A solar salamander". Nature: news.2010.384. doi:10.1038/news.2010.384. ISSN 0028-0836.
- ^ Frazer, Jennifer (May 18, 2018). "Algae Living inside Salamanders Aren't Happy about the Situation". Scientific American Blog Network.
- ^ Burns, John A; Zhang, Huanjia; Hill, Elizabeth; Kim, Eunsoo; Kerney, Ryan (2 May 2017). "Transcriptome analysis illuminates the nature of the intracellular interaction in a vertebrate-algal symbiosis". eLife. 6. doi:10.7554/eLife.22054. PMC 5413350. PMID 28462779.
- ^ Compère, Pierre (November 1999). "Report of the Committee for Algae: 6". Taxon. 48 (1): 135–136. JSTOR 1224630.
- ^ Plotkin, Hod, Zaban; et al. (2010). "Solar energy harvesting in the epicuticle of the oriental hornet (Vespa orientalis)". Naturwissenschaften. 97 (12): 1067–1076. Bibcode:2010NW.....97.1067P. doi:10.1007/s00114-010-0728-1. PMID 21052618. S2CID 14022197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Djeghri, Nicolas; Pondaven, Philippe; Stibor, Herwig; Dawson, Michael N. (2019). "Review of the diversity, traits, and ecology of zooxanthellate jellyfishes" (PDF). Marine Biology. 166 (11): 147. Bibcode:2019MarBi.166..147D. doi:10.1007/s00227-019-3581-6. S2CID 208553146.
- ^ Libes, Susan M. (2009). Introduction to marine biogeochemistry (2 ed.). Academic Press. p. 192. ISBN 978-0-7637-5345-0.
- ^ Dworkin, Martin (2006). The Prokaryotes: Ecophysiology and biochemistry. Vol. 2 (3rd ed.). Springer. p. 988. ISBN 978-0-387-25492-0.
- ^ Lengeler, Joseph W.; Drews, Gerhart; Schlegel, Hans Günter (1999). Biology of the Prokaryotes. Georg Thieme Verlag. p. 238. ISBN 978-3-13-108411-8.
- ^ Bartosik D, Sochacka M, Baj J (July 2003). "Identification and Characterization of Transposable Elements of Paracoccus pantotrophus". J Bacteriol. 185 (13): 3753–63. doi:10.1128/JB.185.13.3753-3763.2003. PMC 161580. PMID 12813068.
- ^ Friedrich, Cornelius G.; et al. (2007). "Redox Control of Chemotrophic Sulfur Oxidation of Paracoccus pantotrophus". Microbial Sulfur Metabolism. Springer. pp. 139–150.[permanent dead link] PDF[dead link]
- ^ a b Stoecker, Diane K. (1998). "Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications". European Journal of Protistology. 34 (3): 281–290. doi:10.1016/S0932-4739(98)80055-2.
- ^ a b Jones, Harriet (1997). "A classification of mixotrophic protists based on their behaviour". Freshwater Biology. 37 (1): 35–43. Bibcode:1997FrBio..37...35J. doi:10.1046/j.1365-2427.1997.00138.x.
- ^ a b c d Mitra, Aditee; Flynn, Kevin J.; Tillmann, Urban; Raven, John A.; Caron, David; Stoecker, Diane K.; Not, Fabrice; Hansen, Per J.; Hallegraeff, Gustaaf; Sanders, Robert; Wilken, Susanne; McManus, George; Johnson, Mathew; Pitta, Paraskevi; Våge, Selina; Berge, Terje; Calbet, Albert; Thingstad, Frede; Jeong, Hae Jin; Burkholder, Joann; Glibert, Patricia M.; Granéli, Edna; Lundgren, Veronica (2016). "Defining Planktonic Protist Functional Groups on Mechanisms for Energy and Nutrient Acquisition: Incorporation of Diverse Mixotrophic Strategies". Protist. 167 (2): 106–120. doi:10.1016/j.protis.2016.01.003. hdl:10261/131722. PMID 26927496.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Tarangkoon, Woraporn (29 April 2010). "Mixtrophic Protists among Marine Ciliates and Dinoflagellates: Distribution, Physiology and Ecology" (PDF). Thesis.[permanent dead link]
External links
[edit]- Troost TA, Kooi BW, Kooijman SA (February 2005). "When do mixotrophs specialize? Adaptive dynamics theory applied to a dynamic energy budget model". Math Biosci. 193 (2): 159–82. doi:10.1016/j.mbs.2004.06.010. PMID 15748728.
- Sanders, Robert W. Mixotrophic Nutrition of Phytoplankton: Venus Fly Traps of the microbial world Archived 2011-08-05 at the Wayback Machine. Temple University.