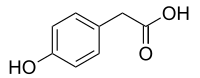
4-Hydroxyphenylacetic acid
Appearance
(Redirected from P-hydroxyphenylacetic acid)
| |
| Names | |
|---|---|
| Preferred IUPAC name
(4-Hydroxyphenyl)acetic acid | |
| Other names
p-Hydroxyphenylacetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1448766 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.321 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O3 | |
| Molar mass | 152.149 g·mol−1 |
| Appearance | Beige powder |
| Melting point | 150 °C (302 °F; 423 K) |
| Hazards | |
| Safety data sheet (SDS) | MSDS at Sigma Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Hydroxyphenylacetic acid is a chemical compound found in olive oil[1] and beer.[2]
Synthesis
[edit]4-Hydroxyphenylacetic acid is obtained by reducing 4-hydroxymandelic acid with elemental phosphorus and iodine.[3]
Uses
[edit]In industry, 4-hydroxyphenylacetic acid is an intermediate used to synthesize atenolol,[3] 3,4-dihydroxyphenylacetic acid,[4] and coclaurine.
References
[edit]- ^ Papadopoulos, George; Boskou, Dimitrios (1991). "Antioxidant effect of natural phenols on olive oil". Journal of the American Oil Chemists' Society. 68 (9): 669. doi:10.1007/BF02662292. S2CID 85234954.
- ^ Determination of free and bound phenolic acids in beer. M. Nardini and A. Ghiselli, Food Chemistry, January 2004, Volume 84, Issue 1, Pages 137–143, doi:10.1016/S0308-8146(03)00257-7
- ^ a b Mattioda, Georges; Christidis, Yani (2000). Glyoxylic Acid. p. 2. doi:10.1002/14356007.a12_495. ISBN 978-3527306732.
{{cite book}}:|journal=ignored (help) - ^ Sutton, Peter; Whittall, John (2012). Practical Methods for Biocatalysis and Biotransformations 2. Chichester, West Sussex: John Wiley & Sons, Ltd. pp. 150–153. ISBN 9781119991397.
