Quenching (fluorescence)

In chemistry, quenching refers to any process which decreases the fluorescent intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisions. As a consequence, quenching is often heavily dependent on pressure and temperature. Molecular oxygen, iodine ions and acrylamide[1] are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence.[2][3][4] Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence.
Quenching is made use of in optode sensors; for instance the quenching effect of oxygen on certain ruthenium complexes allows the measurement of oxygen saturation in solution. Quenching is the basis for Förster resonance energy transfer (FRET) assays.[5][6][7] Quenching and dequenching upon interaction with a specific molecular biological target is the basis for activatable optical contrast agents for molecular imaging.[8][9] Many dyes undergo self-quenching, which can decrease the brightness of protein-dye conjugates for fluorescence microscopy,[10] or can be harnessed in sensors of proteolysis.[11]
Mechanisms
[edit]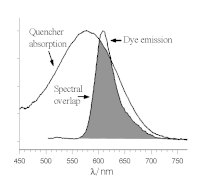
Förster resonance energy transfer
[edit]There are a few distinct mechanisms by which energy can be transferred non-radiatively (without absorption or emission of photons) between two dyes, a donor and an acceptor. Förster resonance energy transfer (FRET or FET) is a dynamic quenching mechanism because energy transfer occurs while the donor is in the excited state. FRET is based on classical dipole-dipole interactions between the transition dipoles of the donor and acceptor and is extremely dependent on the donor-acceptor distance, R, falling off at a rate of 1/R6. FRET also depends on the donor-acceptor spectral overlap (see figure) and the relative orientation of the donor and acceptor transition dipole moments. FRET can typically occur over distances up to 100 Å.
Dexter electron transfer
[edit]Dexter (also known as Dexter exchange or collisional energy transfer, colloquially known as Dexter Energy Transfer) is another dynamic quenching mechanism.[12] Dexter electron transfer is a short-range phenomenon that falls off exponentially with distance (proportional to e−kR where k is a constant that depends on the inverse of the van der Waals radius of the atom[citation needed]) and depends on spatial overlap of donor and quencher molecular orbitals. In most donor-fluorophore–quencher-acceptor situations, the Förster mechanism is more important than the Dexter mechanism. With both Förster and Dexter energy transfer, the shapes of the absorption and fluorescence spectra of the dyes are unchanged.
Dexter electron transfer can be significant between the dye and the solvent especially when hydrogen bonds are formed between them.
Exciplex
[edit]Exciplex (excited state complex) formation is a third dynamic quenching mechanism.

Static quenching
[edit]The remaining energy transfer mechanism is static quenching (also referred to as contact quenching). Static quenching can be a dominant mechanism for some reporter-quencher probes. Unlike dynamic quenching, static quenching occurs when the molecules form a complex in the ground state, i.e. before excitation occurs. The complex has its own unique properties, such as being nonfluorescent and having a unique absorption spectrum. Dye aggregation is often due to hydrophobic effects—the dye molecules stack together to minimize contact with water. Planar aromatic dyes that are matched for association through hydrophobic forces can enhance static quenching. High temperatures and addition of surfactants tend to disrupt ground state complex formation.
Collisional quenching
[edit]Collisional quenching occurs when the excited fluorophore experiences contact with an atom or molecule that can facilitate non-radiative transitions to the ground state. ... Excited-state molecule collides with quencher molecule and returns to ground state non-radiatively.
See also
[edit]- Dark quencher, for use in molecular biology.
- Förster resonance energy transfer, a phenomenon on which some quenching techniques rely
References
[edit]- ^ Phillips SR, Wilson LJ, Borkman RF (August 1986). "Acrylamide and iodide fluorescence quenching as a structural probe of tryptophan microenvironment in bovine lens crystallins". Current Eye Research. 5 (8): 611–9. doi:10.3109/02713688609015126. PMID 3757547.
- ^ O'Reilly JE (September 1975). "Fluorescence experiments with quinine". Journal of Chemical Education. 52 (9): 610–2. Bibcode:1975JChEd..52..610O. doi:10.1021/ed052p610. PMID 1165255.
- ^ Sacksteder L, Ballew RM, Brown EA, Demas JN, Nesselrodt D, DeGraff BA (1990). "Photophysics in a disco: Luminescence quenching of quinine". Journal of Chemical Education. 67 (12): 1065. Bibcode:1990JChEd..67.1065S. doi:10.1021/ed067p1065.
- ^ Gutow JH (2005). "Halide (Cl-) Quenching of Quinine Sulfate Fluorescence: A Time-Resolved Fluorescence Experiment for Physical Chemistry". Journal of Chemical Education. 82 (2): 302. Bibcode:2005JChEd..82..302G. doi:10.1021/ed082p302.
- ^ Peng X, Draney DR, Volcheck WM (2006). "Quenched near-infrared fluorescent peptide substrate for HIV-1 protease assay". In Achilefu S, Bornhop DJ, Raghavachari R (eds.). Optical Molecular Probes for Biomedical Applications. Vol. 6097. pp. 60970F. doi:10.1117/12.669174. S2CID 98507102.
- ^ Peng X, Chen H, Draney DR, Volcheck W, Schutz-Geschwender A, Olive DM (May 2009). "A nonfluorescent, broad-range quencher dye for Förster resonance energy transfer assays". Analytical Biochemistry. 388 (2): 220–8. doi:10.1016/j.ab.2009.02.024. PMID 19248753.
- ^ Osterman H (2009). "The Next Step in Near Infrared Fluorescence: IRDye QC-1 Dark Quencher". Review Article. 388: 1–8. Archived from the original on 20 March 2020.
- ^ Blum G, Weimer RM, Edgington LE, Adams W, Bogyo M (July 2009). "Comparative assessment of substrates and activity based probes as tools for non-invasive optical imaging of cysteine protease activity". PLOS ONE. 4 (7): e6374. Bibcode:2009PLoSO...4.6374B. doi:10.1371/journal.pone.0006374. PMC 2712068. PMID 19636372.
- ^ Weissleder R, Tung CH, Mahmood U, Bogdanov A (April 1999). "In vivo imaging of tumors with protease-activated near-infrared fluorescent probes". Nature Biotechnology. 17 (4): 375–8. doi:10.1038/7933. PMID 10207887. S2CID 12362848.
- ^ Jacobsen MT, Fairhead M, Fogelstrand P, Howarth M (August 2017). "Amine Landscaping to Maximize Protein-Dye Fluorescence and Ultrastable Protein-Ligand Interaction". Cell Chem Biol. 24 (8): 1040–1047. doi:10.1016/j.chembiol.2017.06.015. PMC 5563079. PMID 28757182.
- ^ Voss EW Jr, Workman CJ, Mummert ME (February 1996). "Detection of protease activity using a fluorescence-enhancement globular substrate". BioTechniques. 20 (2): 286–291. doi:10.2144/96202rr06. PMID 8825159.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Dexter excitation transfer (electron exchange excitation transfer)". doi:10.1351/goldbook.D01654
