Ramberg–Bäcklund reaction
| Ramberg–Bäcklund reaction | |
|---|---|
| Named after | Ludwig Ramberg Birger Bäcklund |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | ramberg-baecklund-reaction |
| RSC ontology ID | RXNO:0000094 |
The Ramberg–Bäcklund reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide.[1] The reaction is named after the two Swedish chemists Ludwig Ramberg and Birger Bäcklund. The carbanion formed by deprotonation gives an unstable episulfone that decomposes with elimination of sulfur dioxide. This elimination step is considered to be a concerted cheletropic extrusion.[citation needed]

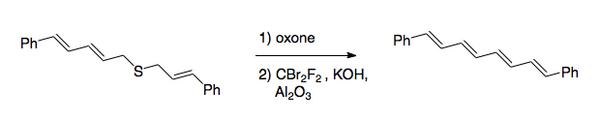
The overall transformation is the conversion of the carbon–sulfur bonds to a carbon–carbon double bond. The original procedure involved halogenation of a sulfide, followed by oxidation to the sulfone. Recently, the preferred method has reversed the order of the steps. After the oxidation, which is normally done with a peroxy acid, halogenation is done under basic conditions by use of dibromodifluoromethane for the halogen transfer step. [2] This method was used to synthesize 1,8-diphenyl-1,3,5,7-octatetraene.
Applications
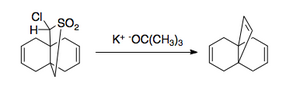
[edit]The Ramberg–Bäcklund reaction has several applications. Due to the nature of elimination, it can be applied to both small rings [3],
and large rings containing a double bond [4].
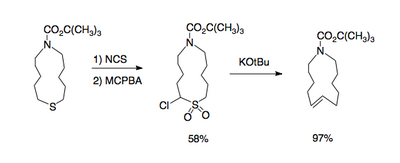
This reaction type gives access to 1,2-dimethylenecyclohexane[5]

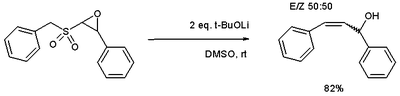
and the epoxide variation [6] access to allyl alcohols.
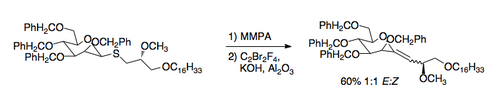
A recently developed application of the Ramberg–Bäcklund reaction is the synthesis of C-glycosides. The required thioethers can be prepared easily by exchange with a thiol. The application of the Ramberg–Bäcklund conditions then leads to an exocyclic vinyl ether that can be reduced to the C-nucleoside [7].
In a variation, oxidation of a sulfamide generates a azo compound.[1]
Substrates
[edit]The necessary α-halo sulfones are accessible through oxidation of the corresponding α-halo sulfides with peracids such as meta-chloroperbenzoic acid; oxidation of sulfides takes place selectively in the presence of alkenes and alcohols. α-Halo sulfides may in turn be synthesized through the treatment of sulfides with halogen electrophiles such as N-chlorosuccinimide or N-bromosuccinimide.[8]
Mechanism
[edit]The sulfone group contains an acidic proton in one of the α-positions which is abstracted by a strong base (scheme 1). The negative charge placed on this position (formally a carbanion) is transferred to the halogen residing on the other α-position in a nucleophilic displacement temporarily forming a three-membered cyclic sulfone. This intermediate is unstable and releases sulfur dioxide to form the alkene. Mixtures of cis isomer and trans isomer are usually obtained.[9]
The Favorskii rearrangement and the Eschenmoser sulfide contraction are conceptually related reactions.
References
[edit]- ^ Ohme, R.; Preuschhof, H.; Heyne, H.-U. (1988). "Azoethane". Organic Syntheses; Collected Volumes, vol. 6, p. 78.
- ^ Ramberg, Ludwig; Bäcklund, Birger (1940). "The reactions of some monohalogen derivatives of diethyl sulfone". Archives of Chemistry, Mineralogy and Geology. 27 (13A): 1–50. ISSN 0365-3781.
- ^ Chan, Tze-Lock; Fong, Sun; Li, Yu; Man, Tim-On; Poon, Chi-Duen (1994). "A new one-flask Ramberg–Bäcklund reaction". Journal of the Chemical Society, Chemical Communications (15): 1771–1772. doi:10.1039/C39940001771.
Cao, Xiao-Ping (2002). "Stereoselective synthesis of substituted all-trans 1,3,5,7-octatetraenes by a modified Ramberg–Bäcklund reaction". Tetrahedron. 58 (7): 1301–1307. doi:10.1016/S0040-4020(01)01224-8. - ^ Paquette, Leo A.; Philips, J. Christopher; Wingard, Robert E. (1971). "α-Halo sulfones. XVIII. Ramberg–Baecklund rearrangement as a synthetic entry to unsaturated propellanes". Journal of the American Chemical Society. 93 (18): 4516–4522. doi:10.1021/ja00747a029.
- ^ Magee, D. I.; Beck, E. J. (August 2000). "The use of the Ramberg–Bäcklund rearrangement for the formation of aza-macrocycles: a total synthesis of manzamine C". Canadian Journal of Chemistry. 78 (8): 1060–1066. doi:10.1139/v00-103.
- ^ Böhme, Horst; Gran, Heinz-Joachim (12 July 1952). "Über die Einwirkung von Chlor auf Thioäther und Mercaptale" [On the action of chlorine on thioether and mercaptals]. Justus Liebigs Annalen der Chemie (in German). 577: 68–77. doi:10.1002/jlac.19525770109.
- ^ Paquette, Leo A. (2005). "The Ramberg–Bäcklund Rearrangement". Organic Reactions. Vol. 25. pp. 1–71. doi:10.1002/0471264180.or025.01. ISBN 9780471264187.
- ^ Block, Eric; Aslam, Mohammad (1987). "A General Synthetic Method for the Preparation of Conjugated Dienes From Olefins Using Bromomethanesulfonyl Bromide: 1,2-Dimethylenecyclohexane". Organic Syntheses. 65: 90. doi:10.15227/orgsyn.065.0090; Collected Volumes, vol. 8, p. 212.
- ^ Evans, P.; Johnson, P.; Taylor, R. J. K. (April 2006). "The Epoxy-Ramberg–Bäcklund Reaction (ERBR): A Sulfone-Based Method for the Synthesis of Allylic Alcohols". European Journal of Organic Chemistry. 2006 (7): 1740–1754. doi:10.1002/ejoc.200500956.
- ^ Griffin, F. K.; Paterson, D. E.; Murphy, P. V.; Taylor, R. J. K. (August 2002). "ChemInform Abstract: A New Route to exo-Glycals Using the Ramberg–Baecklund Rearrangement". ChemInform. 33 (33): 1305. doi:10.1002/chin.200233219.
