Reperfusion therapy
| Reperfusion therapy | |
|---|---|
 Thrombus material (in a cup, upper left corner) removed from a coronary artery during an angioplasty to abort a myocardial infarction. Five pieces of thrombus are shown (arrow heads). |
Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack (myocardial infarction (MI)). Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon and/or stents to open up the artery.[1] Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.
If an MI is presented with ECG evidence of an ST elevation known as STEMI, or if a bundle branch block is similarly presented, then reperfusion therapy is necessary. In the absence of an ST elevation, a non-ST elevation MI, known as an NSTEMI, or an unstable angina may be presumed (both of these are indistinguishable on initial evaluation of symptoms). ST elevations indicate a completely blocked artery needing immediate reperfusion. In NSTEMI the blood flow is present but limited by stenosis. In NSTEMI, thrombolytics must be avoided as there is no clear benefit of their use.[2] If the condition stays stable a cardiac stress test may be offered, and if needed subsequent revascularization will be carried out to restore a normal blood flow. If the blood flow becomes unstable an urgent angioplasty may be required. In these unstable cases the use of thrombolytics is contraindicated.[3]
At least 10% of treated cases of STEMI do not develop necrosis of the heart muscle. A successful restoration of blood flow is known as aborting the heart attack. About 25% of STEMIs can be aborted if treated within the hour of symptoms onset.[4]
Thrombolytic therapy
[edit]Myocardial infarction
[edit]Thrombolytic therapy is indicated for the treatment of STEMI – if it can begin within 12 hours of the onset of symptoms, and the person is eligible based on exclusion criteria, and a coronary angioplasty is not immediately available.[5] Thrombolysis is most effective in the first 2 hours. After 12 hours, the risk of intracranial bleeding associated with thrombolytic therapy outweighs any benefit.[3][6][7] Because irreversible injury occurs within 2–4 hours of the infarction, there is a limited window of time available for reperfusion to work.[citation needed]
Thrombolytic drugs are contraindicated for the treatment of unstable angina and NSTEMI[3][8] and for the treatment of individuals with evidence of cardiogenic shock.[9]
Although no perfect thrombolytic agent exists, ideally it would lead to rapid reperfusion, have a high sustained patency rate, be specific for recent thrombi, be easily and rapidly administered, create a low risk for intracerebral bleeding and systemic bleeding, have no antigenicity, adverse hemodynamic effects, or clinically significant drug interactions, and be cost effective.[10] Currently available thrombolytic agents include streptokinase, urokinase, and alteplase (recombinant tissue plasminogen activator, rtPA). More recently, thrombolytic agents similar in structure to rtPA such as reteplase and tenecteplase have been used. These newer agents boast efficacy at least as well as rtPA with significantly easier administration. The thrombolytic agent used in a particular individual is based on institution preference and the age of the patient.
Depending on the thrombolytic agent being used, additional anticoagulation with heparin or low molecular weight heparin may be of benefit.[11][12] With tPa and related agents (reteplase and tenecteplase), heparin is needed to keep the coronary artery open. Because of the anticoagulant effect of fibrinogen depletion with streptokinase[13] and urokinase[14][15][16] treatment, it is less necessary there.[11]
Failure
[edit]Thrombolytic therapy to abort a myocardial infarction is not always effective. The degree of effectiveness of a thrombolytic agent is dependent on the time since the myocardial infarction began, with the best results occurring if the thrombolytic is used within two hours of the onset of symptoms.[17][18] Failure rates of thrombolytics can be as high as 50%.[19] In cases of failure of the thrombolytic agent to open the infarct-related coronary artery, the person is then either treated conservatively with anticoagulants and allowed to "complete the infarction" or percutaneous coronary intervention (and coronary angioplasty) is then performed.[20] Percutaneous coronary intervention in this setting is known as "rescue PCI" or "salvage PCI". Complications, particularly bleeding, are significantly higher with rescue PCI than with primary PCI due to the action of the thrombolytic.[citation needed]
Side effects
[edit]Intracranial bleeding (ICB) and subsequent stroke is a serious side effect of thrombolytic use. The risk factors for developing intracranial bleeding include a previous episode of intracranial bleed, advanced age of the individual, and the thrombolytic regimen that is being used. In general, the risk of ICB due to thrombolytics is between 0.5 and 1 percent.[11]
Coronary angioplasty
[edit]The benefit of prompt, primary angioplasty over thrombolytic therapy for acute STEMI is now well established.[21][22][23] When performed rapidly, an angioplasty restores flow in the blocked artery in more than 95% of patients compared with the reperfusion rate of about 65% achieved by thrombolysis.[21] Logistic and economic obstacles seem to hinder a more widespread application of angioplasty,[24] although the feasibility of providing regionalized angioplasty for STEMI is currently being explored in the United States.[25] The use of a coronary angioplasty to abort a myocardial infarction is preceded by a primary percutaneous coronary intervention. The goal of a prompt angioplasty is to open the artery as soon as possible, and preferably within 90 minutes of the patient presenting to the emergency room. This time is referred to as the door-to-balloon time. Few hospitals can provide an angioplasty within the 90 minute interval,[26] which prompted the American College of Cardiology (ACC) to launch a national Door to Balloon (D2B) Initiative in November 2006. Over 800 hospitals have joined the D2B Alliance as of March 16, 2007.[27]
One particularly successful implementation of a primary PCI protocol is in the Calgary Health Region under the auspices of the Libin Cardiovascular Institute of Alberta. Under this model, EMS teams responding to an emergency can transmit the ECG directly to a digital archiving system that allows emergency room staff to immediately confirm the diagnosis. This in turn allows for redirection of the EMS teams to those facilities that are ready to conduct time-critical angioplasty. This protocol has resulted in a median time to treatment of 62 minutes.[28]
The current guidelines in the United States restrict angioplasties to hospitals with available emergency bypass surgery as a backup,[5] but this is not the case in other parts of the world.[29]
A PCI involves performing a coronary angiogram to determine the location of the infarcting vessel, followed by balloon angioplasty (and frequently deployment of an intracoronary stent) of the stenosed arterial segment. In some settings, an extraction catheter may be used to attempt to aspirate (remove) the thrombus prior to balloon angioplasty. While the use of intracoronary stents do not improve the short term outcomes in primary PCI, the use of stents is widespread because of the decreased rates of procedures to treat restenosis compared to balloon angioplasty.[30]
Adjuvant therapy during an angioplasty includes intravenous heparin, aspirin, and clopidogrel. Glycoprotein IIb/IIIa inhibitors are often used in the setting of primary angioplasty to reduce the risk of ischemic complications during the procedure.[31][32] Due to the number of antiplatelet agents and anticoagulants used during primary angioplasty, the risk of bleeding associated with the procedure is higher than during an elective procedure.[33]
Coronary artery bypass surgery
[edit]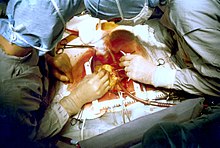
Emergency bypass surgery for the treatment of an acute myocardial infarction (MI) is less common than PCI or thrombolysis. From 1995 to 2004, the percentage of people with cardiogenic shock treated with primary PCI rose from 27.4% to 54.4%, while the increase in coronary artery bypass graft surgery (CABG) was only from 2.1% to 3.2%.[34] Emergency CABG is usually undertaken to simultaneously treat a mechanical complication, such as a ruptured papillary muscle, or a ventricular septal defect, with ensuing cardiogenic shock.[35] In uncomplicated MI, the mortality rate can be high when the surgery is performed immediately following the infarction.[36] If this option is entertained, the patient should be stabilized prior to surgery, with supportive interventions such as the use of an intra-aortic balloon pump.[37] In patients developing cardiogenic shock after a myocardial infarction, both PCI and CABG are satisfactory treatment options, with similar survival rates.[38][39]
Coronary artery bypass surgery involves an artery or vein from the patient being implanted to bypass narrowings or occlusions in the coronary arteries. Several arteries and veins can be used, however internal mammary artery grafts have demonstrated significantly better long-term patency rates than great saphenous vein grafts.[40] In patients with two or more coronary arteries affected, bypass surgery is associated with higher long-term survival rates compared to percutaneous interventions.[41] In patients with single vessel disease, surgery is comparably safe and effective, and may be a treatment option in selected cases.[42] Bypass surgery has higher costs initially, but becomes cost-effective in the long term.[43] A surgical bypass graft is more invasive initially but bears less risk of recurrent procedures (but these may be again minimally invasive).[42]
Reperfusion arrhythmia
[edit]Accelerated idioventricular rhythm which looks like slow ventricular tachycardia is a sign of a successful reperfusion.[44] No treatment of this rhythm is needed as it rarely changes into a more serious rhythm.[45]
See also
[edit]- Perfusion scanning
- Reperfusion injury
- Revascularization
- TIMI
- Ischemia-reperfusion injury of the appendicular musculoskeletal system
References
[edit]- ^ McCoy SS, Crowson CS, Maradit-Kremers H, Therneau TM, Roger VL, Matteson EL, Gabriel SE (May 2013). "Longterm Outcomes and Treatment After Myocardial Infarction in Patients with Rheumatoid Arthritis". The Journal of Rheumatology. 40 (5): 605–10. doi:10.3899/jrheum.120941. PMC 3895921. PMID 23418388.
- ^ "Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial... - PubMed - NCBI". Circulation. 89 (4): 1545–1556. April 1994. doi:10.1161/01.cir.89.4.1545. PMID 8149520.
- ^ a b c Fibrinolytic Therapy Trialists' (FTT) Collaborative Group (1994). "Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients". Lancet. 343 (8893): 311–22. doi:10.1016/s0140-6736(94)91161-4. PMID 7905143.
- ^ Verheugt FW, Gersh BJ, Armstrong PW (2006). "Aborted myocardial infarction: a new target for reperfusion therapy". Eur Heart J. 27 (8): 901–4. doi:10.1093/eurheartj/ehi829. PMID 16543251.
- ^ a b Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr (2004). "ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction)". J Am Coll Cardiol. 44 (3): 671–719. doi:10.1016/j.jacc.2004.07.002. PMID 15358045.
- ^ Boersma E, Maas AC, Deckers JW, Simoons ML (1996). "Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour". Lancet. 348 (9030): 771–5. doi:10.1016/S0140-6736(96)02514-7. hdl:1765/58599. PMID 8813982. S2CID 36710069.
- ^ LATE trial intestigatos. (1993). "Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6-24 hours after onset of acute myocardial infarction". Lancet. 342 (8874): 759–66. doi:10.1016/0140-6736(93)91538-W. PMID 8103874. S2CID 26840289.
- ^ "Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia". Circulation. 89 (4): 1545–56. April 1994. doi:10.1161/01.cir.89.4.1545. PMID 8149520.
- ^ Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH (1999). "Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock". N Engl J Med. 341 (9): 625–34. doi:10.1056/NEJM199908263410901. PMID 10460813.
- ^ White HD, Van de Werf FJ (1998). "Thrombolysis for acute myocardial infarction". Circulation. 97 (16): 1632–46. doi:10.1161/01.cir.97.16.1632. PMID 9593569.
- ^ a b c The GUSTO investigators (1993). "An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators". N Engl J Med. 329 (10): 673–82. doi:10.1056/NEJM199309023291001. hdl:1765/5468. PMID 8204123.
- ^ Sabatine MS, Morrow DA, Montalescot G, Dellborg M, Leiva-Pons JL, Keltai M, Murphy SA, McCabe CH, Gibson CM, Cannon CP, Antman EM, Braunwald E (2005). "Angiographic and clinical outcomes in patients receiving low-molecular-weight heparin versus unfractionated heparin in ST-elevation myocardial infarction treated with fibrinolytics in the CLARITY-TIMI 28 Trial". Circulation. 112 (25). Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators.: 3846–54. doi:10.1161/CIRCULATIONAHA.105.595397. PMID 16291601.
- ^ Cowley MJ, Hastillo A, Vetrovec GW, Fisher LM, Garrett R, Hess ML (1983). "Fibrinolytic effects of intracoronary streptokinase administration in patients with acute myocardial infarction and coronary insufficiency". Circulation. 67 (5): 1031–8. doi:10.1161/01.cir.67.5.1031. PMID 6831667.
- ^ Lourenco DM, Dosne AM, Kher A, Samama M (1989). "Effect of standard heparin and a low molecular weight heparin on thrombolytic and fibrinolytic activity of single-chain urokinase plasminogen activator in vitro". Thromb Haemost. 62 (3): 923–6. doi:10.1055/s-0038-1651029. PMID 2556812.
- ^ Van de Werf F, Vanhaecke J, de Geest H, Verstraete M, Collen D (1986). "Coronary thrombolysis with recombinant single-chain urokinase-type plasminogen activator in patients with acute myocardial infarction". Circulation. 74 (5): 1066–70. doi:10.1161/01.cir.74.5.1066. PMID 2429783.
- ^ Bode C, Schoenermark S, Schuler G, Zimmermann R, Schwarz F, Kuebler W (1988). "Efficacy of intravenous prourokinase and a combination of prourokinase and urokinase in acute myocardial infarction". Am J Cardiol. 61 (13): 971–4. doi:10.1016/0002-9149(88)90108-7. PMID 2452564.
- ^ Morrison LJ, Verbeek PR, McDonald AC, Sawadsky BV, Cook DJ (2000). "Mortality and prehospital thrombolysis for acute myocardial infarction: A meta-analysis". JAMA. 283 (20): 2686–92. doi:10.1001/jama.283.20.2686. PMID 10819952.
- ^ Boersma E, Maas AC, Deckers JW, Simoons ML (1996). "Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour". Lancet. 348 (9030): 771–5. doi:10.1016/S0140-6736(96)02514-7. hdl:1765/58599. PMID 8813982. S2CID 36710069.
- ^ Katz, Richard; Purcell, H. (2006). Acute Coronary Syndromes. Elsevier Health Sciences. p. 96. ISBN 0443102961.
- ^ "Ischemia Reperfusion Research | BMG LABTECH". BMGLabtech.com. Retrieved 2021-11-26.
- ^ a b Keeley EC, Boura JA, Grines CL (2003). "Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials". Lancet. 361 (9351): 13–20. doi:10.1016/S0140-6736(03)12113-7. PMID 12517460. S2CID 9480846.
- ^ Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, Overlie P, Donohue B, Chelliah N, Timmis GC, et al. (1993). "A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group". N Engl J Med. 328 (10): 673–9. doi:10.1056/NEJM199303113281001. PMID 8433725.
- ^ The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. (1997). "A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction". N Engl J Med. 336 (23): 1621–8. doi:10.1056/NEJM199706053362301. hdl:1765/5545. PMID 9173270.
- ^ Boersma E, The Primary Coronary Angioplasty vs. Thrombolysis Group (2006). "Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients". Eur Heart J. 27 (7): 779–88. doi:10.1093/eurheartj/ehi810. PMID 16513663.
- ^ Rokos IC, Larson DM, Henry TD, et al. (2006). "Rationale for establishing regional ST-elevation myocardial infarction receiving center (SRC) networks". Am Heart J. 152 (4): 661–7. doi:10.1016/j.ahj.2006.06.001. PMID 16996830.
- ^ Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, et al. (2006). "Strategies for reducing the door-to-balloon time in acute myocardial infarction". N Engl J Med. 355 (22): 2308–20. doi:10.1056/NEJMsa063117. PMID 17101617. S2CID 1360936.
- ^ "D2B: An Alliance for Quality". American College of Cardiology. 2006. Archived from the original on May 20, 2013. Retrieved April 15, 2007.
- ^ De Villiers JS, Anderson T, McMeekin JD, et al. (2007). "Expedited transfer for primary percutaneous coronary intervention: a program evaluation". CMAJ. 176 (13): 1833–8. doi:10.1503/cmaj.060902. PMC 1891117. PMID 17576980.
- ^ Aversano T, et al. (2002). "Thrombolytic therapy vs primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial". JAMA. 287 (15): 1943–51. doi:10.1001/jama.287.15.1943. PMID 11960536.
- ^ Grines CL, Cox DA, Stone GW, Garcia E, Mattos LA, Giambartolomei A, Brodie BR, Madonna O, Eijgelshoven M, Lansky AJ, O'Neill WW, Morice MC (1999). "Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group". N Engl J Med. 341 (26): 1949–56. doi:10.1056/NEJM199912233412601. PMID 10607811.
- ^ Brener SJ, Barr LA, Burchenal JE, Katz S, George BS, Jones AA, Cohen ED, Gainey PC, White HJ, Cheek HB, Moses JW, Moliterno DJ, Effron MB, Topol EJ (1998). "Randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction. ReoPro and Primary PTCA Organization and Randomized Trial (RAPPORT) Investigators". Circulation. 98 (8): 734–41. doi:10.1161/01.cir.98.8.734. PMID 9727542.
- ^ Tcheng JE, Kandzari DE, Grines CL, Cox DA, Effron MB, Garcia E, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Fahy M, Lansky AJ, Mehran R, Stone GW (2003). "Benefits and risks of abciximab use in primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial". Circulation. 108 (11): 1316–23. doi:10.1161/01.CIR.0000087601.45803.86. PMID 12939213.
- ^ Mukherjee, Debabrata (2006). 900 Questions: An Interventional Cardiology Board Review. Lippincott Williams & Wilkins. ISBN 0-7817-7349-0.
- ^ Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS (2005). "Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock". JAMA. 294 (4): 448–54. doi:10.1001/jama.294.4.448. PMID 16046651.
- ^ Townsend, Courtney M.; Beauchamp D.R.; Evers M.B.; Mattox K.L. (2004). Sabiston Textbook of Surgery - The Biological Basis of Modern Surgical Practice. Philadelphia, Pennsylvania: Elsevier Saunders. p. 1871. ISBN 0-7216-0409-9.
- ^ Kaul TK, Fields BL, Riggins SL, Dacumos GC, Wyatt DA, Jones CR (1995). "Coronary artery bypass grafting within 30 days of an acute myocardial infarction". Ann. Thorac. Surg. 59 (5): 1169–76. doi:10.1016/0003-4975(95)00125-5. PMID 7733715.
- ^ Creswell LL, Moulton MJ, Cox JL, Rosenbloom M (1995). "Revascularization after acute myocardial infarction". Ann. Thorac. Surg. 60 (1): 19–26. doi:10.1016/s0003-4975(95)00351-7. PMID 7598589.
- ^ White HD, Assmann SF, Sanborn TA, et al. (2005). "Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock: results from the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial". Circulation. 112 (13): 1992–2001. doi:10.1161/CIRCULATIONAHA.105.540948. PMID 16186436.
- ^ Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, Col J, White HD (2006). "Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction". JAMA. 295 (21): 2511–5. doi:10.1001/jama.295.21.2511. PMC 1782030. PMID 16757723.
- ^ Raja SG, Haider Z, Ahmad M, Zaman H (2004). "Saphenous vein grafts: to use or not to use?". Heart Lung Circ. 13 (4): 403–9. doi:10.1016/j.hlc.2004.04.004. PMID 16352226.
- ^ Hannan EL, Racz MJ, Walford G, et al. (2005). "Long-term outcomes of coronary-artery bypass grafting versus stent implantation". N. Engl. J. Med. 352 (21): 2174–83. doi:10.1056/NEJMoa040316. PMID 15917382. S2CID 18308025.
- ^ a b Bourassa MG (2000). "Clinical trials of coronary revascularization: coronary angioplasty vs. coronary bypass grafting". Curr. Opin. Cardiol. 15 (4): 281–6. doi:10.1097/00001573-200007000-00013. PMID 11139092. S2CID 36950994.
- ^ Hlatky MA, Boothroyd DB, Melsop KA, et al. (2004). "Medical costs and quality of life 10 to 12 years after randomization to angioplasty or bypass surgery for multivessel coronary artery disease". Circulation. 110 (14): 1960–6. doi:10.1161/01.CIR.0000143379.26342.5C. PMID 15451795.
- ^ Osmancik PP, Stros P, Herman D (2008). "In-hospital arrhythmias in patients with acute myocardial infarction - the relation to the reperfusion strategy and their prognostic impact". Acute Cardiac Care. 10 (1): 15–25. doi:10.1080/17482940701474478. PMID 17924228. S2CID 24957172.
- ^ Dalzell JR, Jackson CE (April 2009). "When the rhythm makes the diagnosis". The Journal of Emergency Medicine. 41 (2): 182–4. doi:10.1016/j.jemermed.2009.02.028. PMID 19345050.
