Salvianolic acids

| |
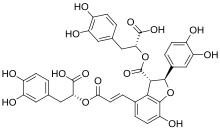
| |

| |
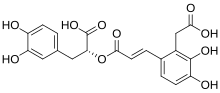 Salvianolic acids A-D
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL | |
| ChemSpider |
|
| DrugBank |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Salvianolic acids are a group of polyphenolic acids consisting on several combinations of caffeic acid and danshensu (salvianic acid) through ester and enol bonds. Salvianolic acids are water-soluble components produced by many species of the genus Salvia, mainly extracted from Salvia miltiorrhiza.[1]
Structural properties
[edit]More than 10 distinct salvianolic acids (Sal), identified by letters from A to J, have been recognized. Notably, Sal-A and Sal-B are the most abundant forms. The combination of danshensu and a caffeic acid derivative or caffeic acid dimer produces several kinds of skeletons. Sal-A is formed by a molecule of danshensu and a dimer of caffeic acid. Sal-B, on the other hand, is constituted by three molecules of danshensu and one molecule of caffeic acid. Sal-C results from the combination of two molecules of danshensu, while Sal-D is characterized as a dimer of caffeic acid.[2]
Biosynthesis and natural occurrence
[edit]The formation of salvianolic acids is closely linked to that of rosmarinic acid. They are primarily synthesized through the phenylpropanoid and tyrosine-derived branches of the phenolic acid biosynthetic pathway. Rosmarinic acid undergoes a not well-understood oxidative reaction to convert into Salvianolic acid B.[3] This reaction involves laccases, which are widely spread in many genera such as Arabidopsis, poplars, sorghum, etc., but appears to be highly expressed in Salvia miltiorrhiza and Salvia bowleyana.[4]
References
[edit]- ^ Du, Guanhua; Song, Junke; Du, Lida; Zhang, Li; Qiang, Guifen; Wang, Shoubao; Yang, Xiuying; Fang, Lianhua (2020-01-01), Du, Guanhua (ed.), "Chapter One - Chemical and pharmacological research on the polyphenol acids isolated from Danshen: A review of salvianolic acids", Advances in Pharmacology, Pharmacological Advances in Natural Product Drug Discovery, 87, Academic Press: 1–41, doi:10.1016/bs.apha.2019.12.004, PMID 32089230, S2CID 211263286, retrieved 2024-01-17
- ^ Ma, Lunkun; Tang, Liling; Yi, Qian (2019). "Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer". Frontiers in Pharmacology. 10: 97. doi:10.3389/fphar.2019.00097. ISSN 1663-9812. PMC 6391314. PMID 30842735.
- ^ Zhou, Zheng; Li, Qing; Xiao, Liang; Wang, Yun; Feng, Jingxian; Bu, Qitao; Xiao, Ying; Hao, Kai; Guo, Meili; Chen, Wansheng; Zhang, Lei (2021-03-18). "Multiplexed CRISPR/Cas9-Mediated Knockout of Laccase Genes in Salvia miltiorrhiza Revealed Their Roles in Growth, Development, and Metabolism". Frontiers in Plant Science. 12: 647768. doi:10.3389/fpls.2021.647768. ISSN 1664-462X. PMC 8014014. PMID 33815454.
- ^ Li, Qing; Feng, Jingxian; Chen, Liang; Xu, Zhichao; Zhu, Yingjie; Wang, Yun; Xiao, Ying; Chen, Junfeng; Zhou, Yangyun; Tan, Hexin; Zhang, Lei; Chen, Wansheng (2019). "Genome-Wide Identification and Characterization of Salvia miltiorrhiza Laccases Reveal Potential Targets for Salvianolic Acid B Biosynthesis". Frontiers in Plant Science. 10: 435. doi:10.3389/fpls.2019.00435. ISSN 1664-462X. PMC 6463009. PMID 31024599.
