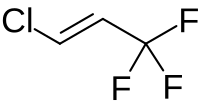
1-Chloro-3,3,3-trifluoropropene
Appearance
(Redirected from Trans-1-Chloro-3,3,3-trifluoropropene)
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-3,3,3-trifluoroprop-1-ene | |
| Other names
1-Chloro-3,3,3-trifluoropropene
HCFO-1233zd HCFC-1233zd r-1233zd Solstice zd (Honeywell brand name) | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.215.615 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H2ClF3 | |
| Molar mass | 130.49 g·mol−1 |
| Appearance | Colorless liquid |
| Boiling point | 39.6 °C (Z) 18.5 °C (E) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Chloro-3,3,3-trifluoropropene (HFO-1233zd) is the unsaturated chlorofluorocarbon with the formula HClC=C(H)CF3. The compound exists as E- (cis-) and Z- (trans-) isomers. The trans- isomer of this colorless gas is of interest as a more environmentally friendly (lower GWP; global warming potential) refrigerant in air conditioners.[1] It is prepared by fluorination and dehydrohalogenation reactions starting with 1,1,1,3,3-pentachloropropane.[2]
References
[edit]- ^ Cheryl Hogue (2011). "Replacing the Replacements". Chemical & Engineering News. 89 (49): 31–32. doi:10.1021/cen-v089n049.p031. Archived from the original on 2020-09-23.
- ^ Tung, Hseuh Sung; Ulrich, Kevin D.; Merkel, Daniel C. "Low-temperature catalytic fluorination process for the manufacture of 1-chloro-3,3,3-trifluoropropene from 1,1,1,3,3-pentachloropropane and hydrogen fluoride" U.S. (2005), US 6844475 B2 20050118 to Honeywell International Business Machines.
