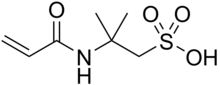
2-Acrylamido-2-methylpropane sulfonic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-2-(prop-2-enamido)propane-1-sulfonic acid | |
| Other names
2-Acrylamido-2-methylpropane sulfonic acid; 2-Acrylamido-2-methylpropanesulfonic acid; 2-Acrylamido-2-methyl-1-propane sulfonic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | AMPS |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.035.683 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H13NO4S | |
| Molar mass | 207.24 g·mol−1 |
| Appearance | White crystalline powder or granular particles |
| Density | 1.1 g/cm3 (15.6 °C) |
| Melting point | 195 °C (383 °F; 468 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Acrylamido-2-methylpropane sulfonic acid (AMPS) was a Trademark name by The Lubrizol Corporation. It is a reactive, hydrophilic, sulfonic acid acrylic monomer used to alter the chemical properties of wide variety of anionic polymers. In the 1970s, the earliest patents using this monomer were filed for acrylic fiber manufacturing. Today, there are over several thousands patents and publications involving use of AMPS in many areas including water treatment, oil field, construction chemicals, hydrogels for medical applications, personal care products, emulsion coatings, adhesives, and rheology modifiers.
Production[edit]
AMPS is made by the Ritter reaction of acrylonitrile and isobutylene in the presence of sulfuric acid and water.[1] The recent patent literature[2] describes batch and continuous processes that produce AMPS in high purity (to 99.7%) and improved yield (up to 89%, based on isobutene) with the addition of liquid isobutene to an acrylonitrile / sulfuric acid / phosphoric acid mixture at 40°C.
Properties[edit]
- Hydrolytic and thermal stability: The geminal dimethyl group and the sulfomethyl group combine to sterically hinder the amide functionality and provide both hydrolytic and thermal stabilities to AMPS-containing polymers.[3][4][5][6]
- Polarity and hydrophilicity: The sulfonate group gives the monomer a high degree of hydrophilicity and anionic character at wide range of pH. In addition, AMPS is absorbing water readily and also imparts enhanced water absorption and transport characteristics to polymers.[7]
- Solubility: AMPS is very soluble in water and dimethylformamide (DMF) and also shows limited solubility in most polar organic solvents.[8]
| Solvent | Solubility (gAMPS/100 g solvent) |
|---|---|
| Water | 150 |
| Dimethylformamide | >100 |
| N-Methyl-2-pyrrolidone | 80 |
| Methanol | 8.7 |
- Inhibition of divalent cation precipitation: Sulfonic acid in AMPS is a very strong ionic group and ionizes completely in aqueous solutions. In applications where the precipitation of mineral salts is undesirable, the incorporation of a polymer containing even a small quantity of AMPS can significantly inhibit the precipitation of divalent cations. The result is a significant reduction in the precipitation of a wide variety of mineral salts, including calcium, magnesium, iron, aluminium, zinc, barium and chromium.[9][10]
- Determining viscosity-average molecular weight (Mark-Houwink constants)[11]
| Na-AMPS | 0.01N | 0.05N | 0.1N | 0.5N | 1.0N | 5.0N |
|---|---|---|---|---|---|---|
| K x 105 | 0.67 | 1.47 | 1.67 | 1.32 | 3.34 | 5.01 |
| ν | 1.02 | 0.91 | 0.88 | 0.86 | 0.77 | 0.72 |
- Reactivity ratio:[12] AMPS reacts well with a variety of vinyl monomers. M2= AMPS or † sodium salt of AMPS
| M1 | r1 | r2 | Remark |
|---|---|---|---|
| Acrylonitrile | 1.2 | 0.7 | DMF |
| Acrylic acid | 0.74 | 0.19 | Water, pH=7.0 |
| Acrylic acid | 1.58 | 0.11 | Water, pH=2~4 |
| Itaconic acid | 0.46 | 0.04 | DMF, 70 °C, Benzoyl Peroxide |
| Acrylamide | 0.98 | 0.49 † | Water, K2S2O8 |
| Styrene | 1.13 | 0.31 | DMF, 60 °C, AIBN |
| Vinyl Acetate | 0.05 | 11.60 † | Methanol, 60 °C, |
| N-Vinylpyrrolidone | 0.13 | 0.66 † | 60 °C, AIBN |
| 2-hydroxyethyl methacrylate | 0.86 | 0.90 | Water, 60 °C, AIBN |
| 2-Hydroxypropyl methacrylate | 6.30 | 0.04 | Water, 80 °C, (NH4)2S2O8 |
| N,N-Dimethylacrylamide | 1.26 | 0.68 † | Water, 30 °C, K2S2O8 |
| N-Vinylformamide | 0.32 | 0.39 † | VA-044 |
Applications[edit]
- Acrylic fiber: A number of enhanced performance characteristics are imparted to acrylic, modified-acrylic, polypropylene and polyvinylidene fluoride fibers: dye receptivity, moisture absorbency, and static resistance.[13]
- Coating and adhesive: Its sulfonic acid group gives the monomers ionic character over a wide range of pH. Anionic charges from AMPS fixed on polymer particles enhance the chemical and shear stabilities of polymer emulsion and also reduce the amount of surfactants leaching out of paint film.[14][15][16] It improves the thermal and mechanical properties of adhesives, and increases the adhesive strength of pressure-sensitive adhesive formulations.[17]
- Detergents: Enhances the washing performance of surfactants by binding multivalent cations and reducing dirt attachment.[18]
- Personal care: Strong polar and hydrophilic properties introduced to a high molecular weight AMPS homopolymer are exploited as a very efficient lubricant characteristic for skin care.[19][20]
- Medical hydrogel: High water-absorbing and swelling capacity when AMPS is introduced to a hydrogel are keys to medical applications. Hydrogel with AMPS showed uniform conductivity, low electrical impedance, cohesive strength, appropriate skin adhesion, and biocompatible and capable of repeated use and have been used to electrocardiograph (ECG) electrodes, defibrillation electrode, electrosurgical grounding pads, and iontophoretic drug delivery electrodes.[21][22][23] In addition, polymers derived from AMPS are used as the absorbing hydrogel and the tackifier component of wound dressings.[20][24][25] Is used due to its high water absorption and retention capability as a monomer in superabsorbents e. g. for baby diapers.[26]
- Oil field applications: Polymers in oil field applications have to stand hostile environments and require thermal and hydrolytic stability and the resistance to hard water containing metal ions. For example, in drilling operations where conditions of high salinity,[27] high temperature and high pressure are present, AMPS copolymers can inhibit fluid loss and be used in oil field environments as scale inhibitors, friction reducers and water-control polymers, and in polymer flooding applications.
- Water treatment applications: The cation stability of the AMPS-containing polymers are very useful for water treatment processes. Such polymers with low molecular weights cannot only inhibit calcium, magnesium, and silica scale in cooling towers and boilers, but also help corrosion control by dispersing iron oxide. When high molecular weight polymers are used, they can be used to precipitate solids in the treatment of industrial effluent stream.[28][29]
- Crop protection: increases in dissolved[30] and nanoparticulate[31] polymer formulations bioavailability of pesticides in aqueous-organic formulations.
- Membranes: It increases water flow, retention and fouling resistance of asymmetric ultrafiltration and microfiltration membranes[32] and is being studied as an anionic component in polymer fuel cell membranes.[33][34]
- Construction applications: Superplasticizers with AMPS are used to reduce water in concrete formulations. Benefits of these additives include improved strength, improved workability, improved durability of cement mixtures.[35] Redispersible polymer powder, when AMPS is introduced, in cement mixtures control air pore content and prevent agglomeration of powders during the spray-drying process from the powder manufacturing and storage.[36] Coating formulations with AMPS-containing polymers prevent calcium ions from being formed as lime on concrete surface and improve the appearance and durability of coating.[37]
See also[edit]
References[edit]
- ^ US 3506707 "Preparation of acrylamidoalkanesulfonic acids." Inventors: Leonard E Miller, Donald L Murfin
- ^ US 6504050 " Process for the preparation of 2-acrylamido-2-methyl-1-propanesulfonic acid." Inventors: P.P. Barve et al.
- ^ W. O. Parker Jr and A. Lezzi, Polymer, 34(23), 4913 (1993)
- ^ Aggour, Y. A. (1994-01-01). "Thermal degradation of copolymers of 2-acrylamido-2-methylpropanesulphonic acid with acrylamide". Polymer Degradation and Stability. 44 (1): 71–73. doi:10.1016/0141-3910(94)90034-5. ISSN 0141-3910.
- ^ Aggour, Y. A. (1998-01-01). "Investigation of the thermal degradation and stability of copolymers of 2-acrylamido-2-methylpropanesulphonic acid and methyl methacrylate". Polymer Degradation and Stability. 60 (2): 317–320. doi:10.1016/S0141-3910(97)00085-2. ISSN 0141-3910.
- ^ Y. A. Aggour, "Copolymerization and thermal investigation of 2-acrylamido-2-methylpropane sulfonic acid with acrylonitrile", J. Polym. Mater. 17, 193 (2000)
- ^ Atta, Ayman M. (August 2002). "Swelling behaviors of polyelectrolyte hydrogels containing sulfonate groups". Polymers for Advanced Technologies. 13 (8): 567–576. doi:10.1002/pat.226. ISSN 1042-7147.
- ^ Lubrizol technical bulletin
- ^ Liu, Yang; Xie, Jian-Jun; Zhang, Xin-Ying (2003-12-20). "Synthesis and properties of the copolymer of acrylamide with 2-acrylamido-2-methylpropanesulfonic acid". Journal of Applied Polymer Science. 90 (13): 3481–3487. doi:10.1002/app.13003. ISSN 0021-8995.
- ^ Rivas, Bernabé L.; Pooley, S. Amalia; Luna, Maribel; Geckeler, Kurt E. (2001-10-03). "Synthesis of water-soluble polymers containing sulfonic acid and amine moieties for the recovery of metal ions using ultrafiltration". Journal of Applied Polymer Science. 82 (1): 22–30. doi:10.1002/app.1819. ISSN 0021-8995.
- ^ J. S. Tan, L. W. Fisher, and P. Markus, "Effects of polymer structure on dilute-solution properties", ACS National Meeting in Philadelphia, Division of Organic Coating and Plastics Preprints, 35(1), 348 (1975)
- ^ Lubrizol Specialty Monomers technical brochure
- ^ EP application 1611278 W. Brennich et al., Applicant: CHT R. Beitlich GmbG, filing date 24. January 2007
- ^ H. Kim, G. P. Marks, and C. Piedrahita, "Superior latex stability and enhanced control of paint rheology using sodium 2-acrylamido-2-methylpropane sulfonate", 236th ACS National Meeting in Philadelphia, Polymeric Materials Science and Engineering Preprint 99, 795 (2008)
- ^ Yang, Yu-Kun; Li, Hao; Wang, Feng (January 2003). "Studies on the water resistance of acrylic emulsion pressure-sensitive adhesives (PSAs)". Journal of Adhesion Science and Technology. 17 (13): 1741–1750. doi:10.1163/156856103322538651. ISSN 0169-4243. S2CID 93181237.
- ^ EP 0973807 Inventor: R. Figge, H.-P. Weitzel, Applicant: Wacker-Chemie GmbH, registered at 20. September 2000
- ^ US 4012560 Inventor: J.C. Baatz, A.E. Corey, Applicant: Monsanto Co., registered at 15. March 1977 and WO 2007/057333, Inventor: A. Hashemzadeh, Applicant: Wacker Polymer Systems, registered at 24. Mai 2007
- ^ US 7928047 Inventor: M.-S. Cho, Applicant: LG Household & Health Care Ltd., registered at 19. April 2011
- ^ US 4128631 "Method of imparting lubricity to keratinous substrates and mucous membranes"
- ^ a b M. Löffler et al., Applicant: Clariant GmbH, registered at 4. September 2002 und EP 2 055 315, Inventor: R. von Eben-Worlée et al., Applicant: Worlée-Chemie, registered at 6. May 2009 EP1236464 Inventor: M. Löffler et al., Applicant: Clariant GmbH, registered at 4. September 2002 und EP 2 055 315, Inventor: R. von Eben-Worlée et al., Applicant: Worlée-Chemie, registered at 6. May 2009
- ^ US 4581821 "Method of preparing tape electrode"
- ^ US 4593053 "Hydrophilic pressure sensitive biomedical adhesive composition"
- ^ US 4768523 "Hydrogel adhesive"
- ^ US 4759354 "Wound dressing"
- ^ G. P. Marks, "A review of the benefits of polymers based on 2-acrylamido-2-methylpropane sulphonic acid, AMPS in medical applications: coatings, electrodes, wound care and dermal delivery systems", European Coatings Conference, Medical Coatings and Adhesives 2008
- ^ WO 2011/131526, Inventor: N. Herfert et al., Applicant: BASF SE, registered at 27. October 2011
- ^ Lubrizol, AMPS(R) Specialty Monomers, Oil Field Applications
- ^ Z. Amjad and R. W. Zhul, "particle size and microscopic investigation of iron oxide foulants in the presence of dispersants", Association of Water technologies, Inc. 18th Annual Convention & Exposition, Charlotte, NC(2006)
- ^ Amjad, Z. (2007-08-01). "Influence of Polymer Architecture on the Stabilization of Iron and Manganese Ions in Aqueous Systems". Tenside Surfactants Detergents. 44 (4): 202–208. doi:10.3139/113.100339. ISSN 2195-8564. S2CID 53546601.
- ^ Patent US20110166309: Preparation containing at least one type of fungicidal conazole. Registered at 14. März 2011, registered at 7. July 2011, Applicant: BASF, Inventor: Sebastian Koltzenburg et al.
- ^ EP 1 681 923, Inventor: S. Koltzenburg et al., Applicant: BASF AG, registered at 20. April 2011
- ^ US 6183640 Inventor: I. Wang, Applicant: USF Filtration and Separations Group, Inc., registered at 6. February 2001
- ^ US 2008/020255 Inventor: H. Hiraoka, T. Yamaguchi, Applicant: Toagosei Co., Ltd., registered at 24. Januar 2008
- ^ Diao, Hanbin; Yan, Feng; Qiu, Lihua; Lu, Jianmei; Lu, Xinhua; Lin, Bencai; Li, Qing; Shang, Songmin; Liu, Wenming (2010-08-10). "High Performance Cross-Linked Poly(2-acrylamido-2-methylpropanesulfonic acid)-Based Proton Exchange Membranes for Fuel Cells". Macromolecules. Vol. 43, no. 15. pp. 6398–6405. doi:10.1021/ma1010099. ISSN 0024-9297.
- ^ Liao, Tung-Sheng; Hwang, Chao-Lung; Ye, Yi-Shian; Hsu, Kung-Chung (April 2006). "Effects of a carboxylic acid/sulfonic acid copolymer on the material properties of cementitious materials". Cement and Concrete Research. 36 (4): 650–655. doi:10.1016/j.cemconres.2005.10.005.
- ^ L.M. Saija and M.Uminski, "Water redispersible acrylic powders for the modification of hydraulic binder compositions" J. Appl. Polym .Sci. 71, 1781 (1999), doi:10.1002/(SICI)1097-4628(19990314)71:11<1781::AID-APP7>3.0.CO;2-2.
- ^ US 6569970 "Preservation of a mineral molding."
- Composition analysis of AMPS-vinyl amide copolymers. J.polym. materials 8(1991)287-289
- Synthesis and characterization of AMPS copolymers with vinylamides by Solution and thermal studies J.polym. materials 11(1994) 207-210.
- High Temperature Molecular relaxations in EMA-AMPS copolymers. recent advances in Polymer Science. Vol.-II 879-(1994)allied publishers.
- Thermoluminescence in probing molecular relaxations and degradation studies of MMA-AMPS copolymers. Bulletin of Electro Chemistry 9(2 & 3) feb. March 1993,pp. 143–145

