N-Hydroxyphthalimide

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxy-1H-isoindole-1,3(2H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.600 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H5NO3 | |
| Molar mass | 163.132 g·mol−1 |
| Appearance | white to pale yellow crystalline solid |
| Density | 1.64 g/mL |
| Melting point | 233°C |
| Boiling point | 370°C |
| water, polar organic solvents | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
N-Hydroxyphthalimide is the organic compound with the formula C6H4(CO)2NOH. A white or yellow solid, it is a derivative of phthalimide. The compound is as a catalyst in the synthesis of other organic compounds.[1][2] It is soluble in water and organic solvents such as acetic acid, ethyl acetate and acetonitrile.[3]
Occurrence and production[edit]
As described by Lassar Cohn in 1880, N-hydroxyphthalimide was produced from phthaloyl chloride and hydroxylamine hydrochloride in the presence of sodium carbonate.[4]
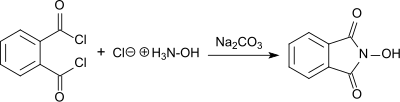
The product forms as a red sodium salt under basic conditions, while white N-hydroxyphthalimide precipitates in 55% yield as the solution is acidified. N-hydroxyphthalimide is also produced by reacting hydroxylamine hydrochloride with diethyl phthalate in the presence of sodium acetate,[5] or with phthalic anhydride in the presence of sodium carbonate with heating. In the last case, an overall yield of 76% is produced following purification by recrystallization.[6]
Microwave irradiation of phthalic anhydride and hydroxylamine hydrochloride in pyridine produces N-hydroxyphthalimide in 81% yield.[7] Even in the absence of a base, phthalic anhydride and hydroxylamine phosphate react to produce N-hydroxyphthalimide in 86% yield when heated to 130 °C.[8]

Properties[edit]
N-Hydroxyphthalimide exists in two polymorphs, colorless and yellow, In the colorless white form, the NOH group is rotated about 1.19° from the plane of the molecule, while in the yellow form it is much closer to planarity (0.06° rotation).[9]
The color of the synthesized N-hydroxyphthalimide is determined by the solvent used; the color transition from white to yellow is irreversible.[10] N-Hydroxyphthalimide forms strongly colored, mostly yellow or red salts with alkali and heavy metals, ammonia and amines.[11] Hydrolysis of N-hydroxyphthalimide by the addition of strong bases produces phthalic acid monohydroxamic acid by adding water across one of the carbon–nitrogen bonds.[5] N-Hydroxyphthalimide ethers, on the other hand, are colorless and provide O-alkylhydroxylamines by alkaline hydrolysis or cleavage through hydrazine hydrate.
The "phthalylhydroxylamine" reported by Cohn was known to have a molecular formula of C
8H
5NO
3, but the exact structure was not known.[4] Three possibilities were discussed and are shown in the Figure below: a mono-oxime of phthalic anhydride ("phthaloxime", I), an expanded ring with two heteroatoms, (2,3-benzoxazine-1,4-dione, II), and N-hydroxyphthalimide (III).[10][12] It was not until the 1950s that Cohn's product was definitely shown to be N-hydroxyphthalimide (III).[13]

8H
5NO
3 considered as Cohn's "phthalylhydroxylamine"
Applications and reactions[edit]
Nefkens and Tesser developed a technique for generating active esters from N-hydroxyphthalimide[14] for use in peptide synthesis,[15] an approach later extended to using N-hydroxysuccinimide.[16] The ester linkage is formed between the N-hydroxyphthalimide and a carboxylic acid by elimination of water, the coupling achieved with N,N′-dicyclohexylcarbodiimide (DCC). For peptide synthesis, the N-terminus of the growing peptide is protected with tert-butyloxycarbonyl while its C-terminus (Z–NH–CH(R)–COOH) is coupled to N-hydroxyphthalimide. An ester of the next amino acid in the desired peptide sequence is shaken with activated ester, adding to the chain and displacing the N-hydroxyphthalimide. This reaction is quantitative and nearly instantaneous at 0 °C.[15][17] The resulting ester needs to be hydrolysed before the cycle can be repeated.

The N-hydroxyphthalimide can be removed by shaking with sodium bicarbonate,[15] but the N-hydroxysuccinimide approach shows greater reactivity and convenience, and is generally preferred.[16][17]
Esters of N-hydroxyphthalimide and activated sulfonic acids such as trifluoromethanesulfonic anhydride or p-toluenesulfonyl chloride are used as so-called photoacids, which split off protons during UV irradiation.

The protons generated serve for the targeted local degradation of acid-sensitive photoresists.[18]
N-Hydroxyphthalimide can be converted with vinyl acetate in the presence of palladium(II)acetate to the N-vinyloxyphthalimide, which is quantitatively hydrogenated to N-ethoxyphthalimide and subsequently O-ethylhydroxylamine.[19]

A variety of functional groups can be oxidized with the aminoxyl radical (phthalimide-N-oxyl, PINO)[20] formed by the abstraction of a hydrogen atom from N-hydroxyphthalimide under gentle conditions (similar to TEMPO):[1]
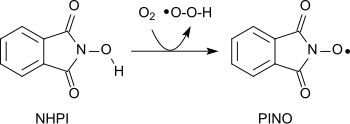
Using molecular oxygen alkanes can be oxidized to form alcohols, secondary alcohols to ketones, acetals to esters and alkenes to epoxides.[21][22][23] Amides can be converted into carbonyl compounds with N-hydroxyphthalimide and cobalt(II)salts under mild conditions.[24]

Efficient oxidation reactions of precursors of important basic chemicals are of particular technical interest. For example, ε-caprolactam can be prepared using NHPI from the so-called KA oil ("ketone-alcohol" oil, a mixture of cyclohexanol and cyclohexanone) which is obtained during the oxidation of cyclohexane. The reaction proceeds via cyclohexanol hydroperoxide, which reacts with ammonia to give peroxydicyclohexylamine followed by a rearrangement in the presence of catalytic amounts of lithium chloride.[22][25]

The use of N-hydroxyphthalimide as a catalyst in the oxidation of KA oil avoids the formation of the undesirable by-product ammonium sulfate which is produced by the conventional ε-caprolactam synthesis (Beckmann rearrangement of cyclohexanone oxime with sulfuric acid).
Alkanes are converted into nitroalkanes in the presence of nitrogen dioxide.[26]
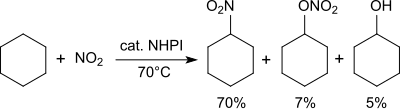
Cyclohexane is converted at 70 °C with nitrogen dioxide/air into a mixture of nitrocyclohexane (70%), cyclohexyl nitrate (7%) and cyclohexanol (5%).
N-hydroxyphthalimide serves as an oxidizing agent in photographic developers[27] and as charge control agents in toners[28] have been described in the patent literature.
Phthalimido-N-oxyl (PINO)[edit]
The radical derived by removal of a hydrogen atom from N-hydroxyphthalimide is called N-phthalimido-N-oxyl, acronym being PINO. It is a powerful H-atom abstracting agent.[1] The bond dissociation energy of NHPI (i.e., PINO–H) is 88–90 kcal/mol (370–380 kJ/mol), depending on the solvent.[29]
References[edit]
- ^ a b c Recupero, Francesco; Punta, Carlo (2007). "Free Radical Functionalization of Organic Compounds Catalyzed by N-Hydroxyphthalimide". Chem. Rev. 107 (9): 3800–3842. doi:10.1021/cr040170k. PMID 17848093.
- ^ Melone, Lucio; Punta, Carlo (2013). "Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review". Beilstein J. Org. Chem. 9: 1296–1310. doi:10.3762/bjoc.9.146. PMC 3701383. PMID 23843925.
- ^ Gambarotti, Cristian; Punta, Carlo; Recupero, Francesco; Zlotorzynska, Maria; Sammis, Glenn (2013). "N-Hydroxyphthalimide". N-Hydrophthalimide. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00598.pub2. ISBN 978-0471936237.
- ^ a b Cohn, Lassar (1880). "Phthalylhydroxylamin: Ueberführung der Phthalsäure in Salicylsäure" [N-hydroxyphthalimide: Conversion of phthalic acid into salicylic acid]. Justus Liebigs Ann. Chem. (in German). 205 (3): 295–314. doi:10.1002/jlac.18802050304.
- ^ a b Bauer, Ludwig; Miarka, Stanley V. (1957). "The Chemistry of N-Hydroxyphthalimide". J. Am. Chem. Soc. 79 (8): 1983–1985. doi:10.1021/ja01565a061.
- ^ Gross, H.; Keitel, I. (1969). "Zur Darstellung von N-Hydroxyphthalimid und N-Hydroxysuccinimid" [On the preparation of N-hydroxyphthalimide and N-hydroxysuccinimide]. J. Prakt. Chem. (in German). 311 (4): 692–693. doi:10.1002/prac.19693110424.
- ^ Sugamoto, Kazuhiro; Matsushita, Yoh‐ichi; Kameda, Yu‐hei; Suzuki, Masahiko; Matsui, Takanao (2005). "Microwave‐assisted Synthesis of N‐Hydroxyphthalimide Derivatives". Synth. Commun. 35 (1): 67–70. doi:10.1081/SCC-200046498. S2CID 96623891.
- ^ EP application 1085013, Elke Fritz-Langhals, "Verfahren zur Herstellung cyclischer N-Hydroxy-dicarboximide [Process for the preparation of cyclic N-hydroxydicarboximides]", published 2001-03-21, assigned to Consortium für elektrochemische Industrie GmbH
- ^ Reichelt, Hendrik; Faunce, Chester A.; Paradies, Henrich H. (2007). "Elusive forms and structures of N-hydroxyphthalimide: The colorless and yellow crystal forms of N-hydroxyphthalimide". J. Phys. Chem. A. 111 (13): 2587–2601. Bibcode:2007JPCA..111.2587R. doi:10.1021/jp068599y. PMID 17388355.
- ^ a b Ames, D. E.; Grey, T. F. (1955). "N-Hydroxy-imides. Part II. Derivatives of homophthalic and phthalic acid". J. Chem. Soc.: 3518–3521. doi:10.1039/JR9550003518.
- ^ Porcheddu, Andrea; Giacomelli, Giampaolo (2009). "Synthesis of oximes and hydroxamic acids". In Rappaport, Zvi; Lieberman, Joel F. (eds.). The Chemistry of Hydroxylamines, Oximes, and Hydroxamic Acids, Part 1. Chichester: Wiley. pp. 224–226. ISBN 978-0-470-51261-6.
- ^ Bradly, Oscar L.; Baker, Leslie C.; Goldstein, Richard F.; Harris, Samuel (1928). "LXVIII.—The isomerism of the oximes. Part XXXIII. The oximes of opianic acid and of phthalic anhydride". J. Chem. Soc.: 529–539. doi:10.1039/JR9280000529.
- ^ Hurd, Charles D.; Buess, Charles M.; Bauer, Ludwig (1954). "Succino- and phthalo-hydroxamic acids". J. Org. Chem. 19 (7): 1140–1149. doi:10.1021/jo01372a021.
- ^ Nefkens, G. H. L.; Tesser, G. I.; Nivard, R. J. F. (1962). "Synthesis and reactions of esters of N-hydroxyphthalimide and N-protected amino acids". Recl. Trav. Chim. Pays-Bas. 81 (8): 683–690. doi:10.1002/recl.19620810807.
- ^ a b c Nefkens, G. H. L.; Tesser, G. I. (1961). "A Novel Activated Ester in Peptide Synthesis". J. Am. Chem. Soc. 83 (5): 1263. doi:10.1021/ja01466a068.
- ^ a b Anderson, George W.; Zimmerman, Joan E.; Callahan, Francis M. (1964). "The Use of Esters of N-Hydroxysuccinimide in Peptide Synthesis". J. Am. Chem. Soc. 86 (9): 1839–1842. doi:10.1021/ja01063a037.
- ^ a b Bodanszky, Miklos (1993). "Activation and Coupling". Principles of Peptide Synthesis (2nd ed.). Springer-Verlag. pp. 9–61. doi:10.1007/978-3-642-78056-1_2. ISBN 9783642780561.
- ^ EP 0919867, K. Elian, E. Günther, R. Leuschner, "Chemisch verstärkter Resist für die Elektronenstrahllithografie", published 2003-05-21, assigned to Infineon Technologies AG
- ^ WO 1995025090, D.M.C. Callant, A.M.C.F. Castelijns, J.G. De Vries, "Cyclic N-alkenyloxyimides and a method for the preparation of cyclic N-alkenyloxyimides, the corresponding cyclic N-alkoxyimides and O-alkoxyamines", published 1995-09-21, assigned to DSM N.V.
- ^ S. Coseri (2009), "Phthalimide‐N‐oxyl (PINO) Radical, a Powerful Catalytic Agent: Its Generation and Versatility Towards Various Organic Substrates", Catal. Rev. Sci. Eng., vol. 51, no. 2, pp. 218–292, doi:10.1080/01614940902743841, S2CID 97018136
- ^ Y. Ishii, K. Nakayama, M. Takeno, S. Sakaguchi, T. Iwahama, Y. Nishiyama (1995), "Novel Catalysis by N-Hydroxyphthalimide in the Oxidation of Organic Substrates by Molecular Oxygen", J. Org. Chem., vol. 60, no. 13, pp. 3934–3935, doi:10.1021/jo00118a002
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b "Discovery of a carbon radical producing catalyst and its application to organic synthesis" (PDF). TCIMAIL, Number 116. Tokyo Chemical Industry Co. Ltd. April 2003. Retrieved 2016-08-11.
- ^ B.B. Wentzel, M.P.J. Donners, P.L. Alsters, M.C. Feiters, R.J.M. Nolte (2000), "N-Hydroxyphthalimide/cobalt(II) catalyzed low temperature benzylic oxidation using molecular oxygen", Tetrahedron, vol. 56, no. 39, pp. 7797–7803, doi:10.1016/S0040-4020(00)00679-7
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ F. Minisci, C. Punta, F. Recupero, F. Fontana, G.F. Pedulli (2002), "Aerobic Oxidation of N-Alkylamides Catalyzed by N-Hydroxyphthalimide under Mild Conditions. Polar and Enthalpic Effects", J. Org. Chem., vol. 67, no. 8, pp. 2671–2676, doi:10.1021/jo016398e, PMID 11950315
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ O. Fukuda, S. Sakaguchi, Y. Ishii (2001), "A new strategy for catalytic Baeyer-Villiger oxidation of KA-oil with molecular oxygen using N-hydroxyphthalimide", Tetrahedron Lett., vol. 42, no. 20, pp. 3479–3481, doi:10.1016/S0040-4039(01)00469-5
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ S. Sakaguchi, Y. Nishiwaki, T. Kitamura, Y. Ishii (2001), "Efficient catalytic alkane nitration with NO2 under air assisted by N-hydroxyphthalmide", Angew. Chem., Int. Edit., vol. 40, no. 1, pp. 222–224, doi:10.1002/1521-3773(20010105)40:1<222::AID-ANIE222>3.0.CO;2-W
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ EP application 0664479, W. Ishikawa & T. Sampei, "Method of processing silver halide photographic lightsensitive material", published 1994-07-26, assigned to Konica Corp.
- ^ US 5332637, J.C. Wilson; S.M. Bonser & H.W. Osterhoudt, "Electrostatographic dry toner and developer compositions with hydroxyphthalimide", issued 1994-07-26, assigned to Eastman Kodak Co.
- ^ Coseri, Sergiu (2009). "Phthalimide‐N‐oxyl (PINO) Radical, a Powerful Catalytic Agent: Its Generation and Versatility Towards Various Organic Substrates". Catalysis Reviews. 51 (2): 218–292. doi:10.1080/01614940902743841. S2CID 97018136.
