Polyoxetane

Polyoxetane (POX), or poly(oxetane), is synthetic organic heteroatomic thermoplastic polymer with molecular formula (–OCH2CH2CH2–)n. It is polymerized from oxetane monomer, which is a four-membered cyclic ether.
History[edit]
Needed chemistry was observed and developed through the 1930s and 1940s. The very first polymerized oxetane was 3,3-bis(chloromethyl)oxetane followed by other 3,3-disubstituted derivatives during the 1950s.[1] Unsubstituted oxetane itself was polymerized in 1956.
Monomers[edit]
Tens of oxetane derivatives have been synthesized and many of them are polymerizable.[2] Reasons for inability to polymerize are different basicity and ring strain caused by different electron and bulkiness of substituents also as their position. Major 3-substituted and 3,3-disubstituted monomers are summarized in the oxetane article.
Polymerization[edit]
Mechanism[edit]

Ring strain of unsubstituted oxetane is 107 kJ/mol.[2] That is twenty[2] times more, than non-polymerizable six-membered tetrahydropyran. Oxetane polymerizes via a cationic, ring-openning mechanism. Special oxetanes are polymerizable by other mechanisms.[3][4]
The propagation centre is a tertiary oxonium ion, mainly initialized by Lewis acids, trialkyl oxonium salts, carbocationic salts and others. Strong acids tend to generate secondary oxonium ions, which are unreactive, thus they are not initiators of first choice. On the other hand super acids (eg. HSO3F) are effective initiators of cationic polymerization of cyclic ethers, such as oxetane. For sufficient stability of propagation centre, a counterion X– of low nucleophylity is required, such as SbCl6–, PF6–, AsF6– or SbF6–.[5][6] First polymerizations were conducted with compounds consisting of BF4– or BF3OH– counterions.[2] Propagation is very fast and thus preparation of lower molecular weight products (also with desired functional end groups) wasnlt performed until today.[when?][2]
Side reactions[edit]
Unsymmetrically substituted oxetanes polymerizes according to ability of attacking one or both alpha-carbons of the propagation centre. Unsubstituted and 3-substituted derivatives polymerize in symmetrical manner, but 2-substituted derivatives can form any of the basic types of polymer chain connections (head-to-tail, head-to-head and tail-to-tail). However, with right conditions and initiation system used, a stereospecific propagation can be achieved.[7]
Oxygen atoms of the main chain possess enough reactivity to attack oxonium propagation centre to either form cyclic oligomers (usually tetramers[8]) or to depolymerize.
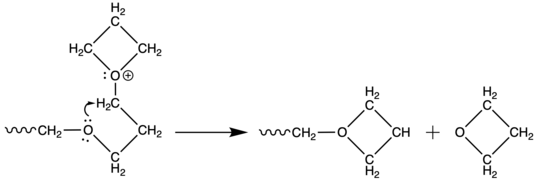
These reactions within one molecule are referred as backbitting. During polymerization of unsubstituted oxetane, mutual attack of two growing chains may occur, in very small number, to form acyclic oxonium ions. This process is so called temporary termination.[9]
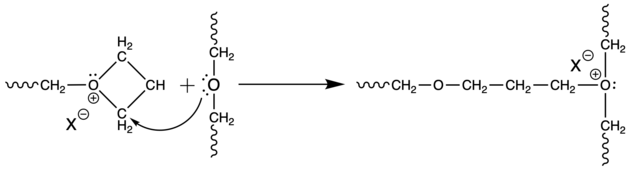
Mentioned side reactions compete in speed with propagation. The faster the propagation, the less side reactions take place. Speed of propagation depends on polymerized monomer, initiation system used and polymerization conditions set.
Example of industrial production[edit]
Polymerization is conducted in mixture of methylene chloride and petrol in -25 °C for 4 to 8 hours to obtain suspension of polymer. Catalytic system consists of 1-2 % BF3 and 0,1-0,4 % epichlorhydrin which acts as a cocatalyst. Final suspension is neutralised, stripped by water steam, filtered, washed and dried.[10]
Substituted polyoxetanes[edit]
A series of substituted oxetanes have been synthesized and polymerized. The very first polymerized oxetane was 3,3-bis(chloromethyl)oxetane.[2]
Properties[edit]
Polyoxetanes can be liquids or solids with high range of crystallinity and melting temperature. Final material characteristics depend on symmetry, bulkiness and polarity of the substituents.[2] For example, melting temperature of POX is 35 °C. One methyl substituent in position 2 or 3 ensures amorphous character of polymethyloxetanes.[11] Oxetanes symmetrically bisubstituted on the same carbon, give crystalline polymers, such as 3,3-dimethyloxetane. Melting point of poly(3,3-dimethyloxetane) is 47 °C. Halogens increase melting point of oxetane polymers. The bigger halogen atom, the higher melting temperature is. Melting temperature of halogenated oxetanes vary from 135 to 290 °C.[11] Amorphous low melting oxetanes are soluble in common organic solvents, on the other hand crystalline are not.[12]
Polymeranalogical reaction[edit]
Butyllithium has been used to break up polyoxetane to lower molecular weight POX glycols with hydroxyl (–OH) functional end groups. With the same result, degradation with ozone followed by reduction by LiAlH4 can be used.[13] Polyoxetane glycols can be used for manufacturing of polyurethane networks[14] and preparation of copolymers.[15]
Copolymers[edit]
Two main reasons to copolymerize oxetanes are adjustment of crystallinity and modification of material properties.
Oxetanes are copolymerized mainly with tetrahydrofuran (THF) to produce precursors of soft segments of polyurethanes (PUR), polyethers and polyamide elastomers.[16] Particularly statistic copolymer of BCMO and THF is amorphous, tough rubber.[17] Unhomopolymerizable derivatives of oxetane are able to copolymerize with homopolymerizable oxetanes. Most studied monomer in copolymerization problemstics have been BCMO.[18] Also copolymers with thermoplastic elastomer behavior have been prepared.[15]
Applications[edit]
Polyoxetanes are engineering polymers. Only one oxetane polymer, derived from 3,3–bis(chloromethyl)oxetane (BCMO) had industrial application. It was available under trade mark Penton by Hercules, Inc. (USA) and Pentaplast (Russia). Main use were sterilizable goods because of relatively high heat-distortion temperature and low water absorption. BCMO is self extinguishing (because of chlorine atoms present in polymer chain) and is highly chemically resistant. It stands up to most organic solvents and strong alkali. It dissolves in strong acids, such as concentrated HNO3 or H2SO4.[19] A typical number-average molecular weight range between 250 000 nad 350 000 g/mol. It can be conventionally processed via injection moulding. Moulded goods exhibit low shrinkage and fantastic dimensional stability in general.[19]
Examples of parts that can be constructed from costly PBCMO are bearings, valves, parts for fitting cables and electrical parts, etc. It is a very good anti-corrosive coating with guarantee of corrosive stability with main use for chemical tanks.[20] It's great material for desalination membranes.[21] Perfluorinated oxetanes (–CF2CF2CF2O–)n exhibit great friction-reducing properties[22] and are potentially useful for gas separation membranes[23]
Significant part of oxetanes are turned into polyoxetanes glycols and other polymeric materials.[24]
Energetic polymers[edit]
By replacing hydrogen(s) in position 3 by electron deficient groups, energetic polymers can be prepared. Desired functional groups are ethyl (CH3–CH2–), nitro (NO2–) or 2-oxa-4,4-dinitropentyl (CH3–C(NO2)2–CH2–O–CH2–). Energetic polymers can be used as explosives and propellants or they are precursors for manufacturing of mentioned above. They burn with a great deal of smoke.
References[edit]
- ^ Farthing, Alan C.; Reynolds, R. J. William (1954). "Synthesis and properties of a new polyether: Poly-3,3-bis(chloromethyl)-1-oxabutene". Journal of Polymer Science. 12 (1): 503–507. doi:10.1002/pol.1954.120120142. ISSN 0022-3832.
- ^ a b c d e f g Dreyfuss, M. P.; Dreyfuss, P. (15 July 2011), "Oxetane Polymers", in John Wiley & Sons, Inc. (ed.), Encyclopedia of Polymer Science and Technology, Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. pst520, doi:10.1002/0471440264.pst520, ISBN 978-0-471-44026-0, retrieved 5 December 2022
- ^ E. J. Vandenberg and A. E. Robinson in E. J. Vandenberg, ed., Polyethers, ACS Symp, Ser. 6, American Chemical Society, Washington, D.C., 1975, pp. 101–119
- ^ Hirano, Tsuneo; Nakayama, Shinichi; Tsuruta, Teiji (June 1975). "A possibility of anionic polymerization of oxetane by a coordination mechanism". Die Makromolekulare Chemie. 176 (6): 1897–1900. doi:10.1002/macp.1975.021760628. ISSN 0025-116X.
- ^ Dreyfuss, P (1982). Poly(tetrahydrofuran). Gordon & Breach. OCLC 636328269.
- ^ Kops, Jørgen; Hvilsted, Søren; Spanggaard, Hans (September 1980). "Structural Aspects of the Ring-Opening Polymerization of 2-Methyloxacyclobutane". Macromolecules. 13 (5): 1058–1062. doi:10.1021/ma60077a007. ISSN 0024-9297.
- ^ Oguni, Nobuki; Hyoda, Junko (November 1980). "Polymerization of 2-Methyltrimethylene Oxide with Organoaluminum Catalysts and a Microstructure Study of Its Polymer by 13C NMR Spectroscopy". Macromolecules. 13 (6): 1687–1690. doi:10.1021/ma60078a058. ISSN 0024-9297.
- ^ Rose, J. B. (1956). "111. Cationic polymerisation of oxacyclobutanes. Part I". Journal of the Chemical Society (Resumed): 542. doi:10.1039/jr9560000542. ISSN 0368-1769.
- ^ Black, P. E.; Worsfold, D. J. (1 November 1976). "The polymerization of oxetane with hexafluorophosphate salts". Canadian Journal of Chemistry. 54 (21): 3325–3329. doi:10.1139/v76-479. ISSN 0008-4042.
- ^ Katajeva V.M., Popova V.A., Sažina B.I.: Spravočnik po plastmassam. Sv. 1. 2. vyd. Str. 268. Chimia, Moscov 1975.
- ^ a b Goethals, Lucien; Sioen, Philip (2000). "Dialogos. Lucien Goethals in gesprek met Philip Sioen". Revue belge de Musicologie / Belgisch Tijdschrift voor Muziekwetenschap. 54: 49–71. doi:10.2307/3686877. ISSN 0771-6788. JSTOR 3686877.
- ^ Kline, G. M. (16 September 1966). "Chemical Reference Book: Polymer Handbook. J. Brandrup and E. H. Immergut, Eds. Interscience (Wiley), New York, 1966. 1276 pp., illus. $19.50". Science. 153 (3742): 1372. doi:10.1126/science.153.3742.1372-a. ISSN 0036-8075.
- ^ Conjeevaram, S. V.; Benson, R. S.; Lyman, D. J. (February 1985). "Block copolyurethanes based on polyoxytrimethylene glycols". Journal of Polymer Science: Polymer Chemistry Edition. 23 (2): 429–444. doi:10.1002/pol.1985.170230217. ISSN 0360-6376.
- ^ Schmitt, G. C. (May 1985). "Ring-opening polymerization, volumes 1 and 2 and indexes, K. J. Ivin and T. Saegusa, Eds., Elsevier Science, New York, 1984, Vol. 1, 521 pp.; Vol. 2,1131 pp. Price: $277.75". Journal of Polymer Science: Polymer Letters Edition. 23 (5): 275. doi:10.1002/pol.1985.130230513. ISSN 0360-6384.
- ^ a b Hardenstine, K. E.; Murphy, C. J.; Jones, R. B.; Sperling, L. H.; Manser, G. E. (May 1985). "Characterization of block copolymers based on poly[3,3-bis(ethoxymethyl)oxetane] and other novel polyethers". Journal of Applied Polymer Science. 30 (5): 2051–2064. doi:10.1002/app.1985.070300522. ISSN 0021-8995.
- ^ Cabal, Luis A; Reed, John; Miller, Frank; Hodgman, Joan E (April 1981). "120 Elevated Blood Pressure in Infants of Pre-Eclamptic Mothers". Pediatric Research. 15: 459. doi:10.1203/00006450-198104001-00129. ISSN 0031-3998. S2CID 40895078.
- ^ Dreyfuss, M. P.; Dreyfuss, P. (September 1966). "p-Chlorophenyldiazonium hexafluorophosphate as a catalyst in the polymerization of tetrahydrofuran and other cyclic ethers". Journal of Polymer Science Part A-1: Polymer Chemistry. 4 (9): 2179–2200. doi:10.1002/pol.1966.150040913. ISSN 0449-296X.
- ^ Dreyfuss, Patricia; Dreyfuss, M. Peter (1989), "Cationic Ring-opening Polymerization: Copolymerization", Comprehensive Polymer Science and Supplements, Elsevier, pp. 851–860, doi:10.1016/b978-0-08-096701-1.00115-4, ISBN 9780080967011, retrieved 28 December 2022
- ^ a b Kleinschmidt, K. (1 March 1979). "Book Reviews : ACOUSTIC DESIGN AND NOISE CONTROL VOLUME 1, ACOUSTIC DESIGN M. Rettinger Chemical Publishing Co., Inc., New York (1977)". The Shock and Vibration Digest. 11 (3): 27–28. doi:10.1177/058310247901100307. ISSN 0583-1024.
- ^ "КЛИНИЧЕСКАЯ И ЭКСПЕРИМЕНТАЛЬНАЯ МЕДИЦИНА". Proceedings of the National Academy of Sciences of Belarus, Medical Series (in Belarusian). 17 (3). 29 August 2020. doi:10.29235/1814-6023-2020-17-3. ISSN 2524-2350.
- ^ Bittencourt, E.; Stannett, V.; Williams, J. L.; Hopfenberg, H. B. (March 1981). "Novel ion-containing reverse osmosis membranes. I. Preparation and selected properties". Journal of Applied Polymer Science. 26 (3): 879–888. doi:10.1002/app.1981.070260312. ISSN 0021-8995.
- ^ https://www.ssi.shimadzu.com/sites/ssi.shimadzu.com/files/pim/pim_document_file/ssi/others/15911/SafetyDataSheet_220-94824-21.pdf
- ^ Yampolskii, Yu.; Belov, N.; Alentiev, A. (15 March 2020). "Perfluorinated polymers as materials of membranes for gas and vapor separation". Journal of Membrane Science. 598: 117779. doi:10.1016/j.memsci.2019.117779. ISSN 0376-7388. S2CID 214064049.
- ^ "Coughtrie, Thomas, (28 Oct. 1895–29 Oct. 1985), Chairman, Bruce Peebles Industries Ltd, and Bruce Peebles Ltd, Edinburgh, 1961–67", Who Was Who, Oxford University Press, 1 December 2007, doi:10.1093/ww/9780199540884.013.u163177, retrieved 28 December 2022
