Polyoxymethylene dimethyl ethers
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
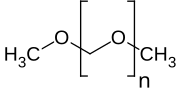
| H3CO(CH2O)nCH3 | |
| Molar mass | Variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polyoxymethylene dimethyl ethers (PODE or DMMn) are a class of chemical compounds with the molecular formula H3CO(CH2O)nCH3 where n is typically about 3 to 8.
PODE can be produced from methylal and formaldehyde or a formaldehyde equivalent such as paraformaldehyde[1] or trioxane.[2]
PODE is used as a diesel fuel additive[3] and as a solvent.
References[edit]
- ^ Arvidson M., Fakley M.E., Spencer M.S. (1987). "Lithium halide-assisted formation of polyoxymethylene dimethyl ethers from dimethoxymethane and formaldehyde". Journal of Molecular Catalysis. 41 (3): 391–393. doi:10.1016/0304-5102(87)80118-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Qi Zhao; Hui Wang; Zhang-feng Qin; Zhi-wei Wu; Jian-bing Wu; Wei-bin Fan; Jian-guo Wang (2011). "Synthesis of polyoxymethylene dimethyl ethers from methanol and trioxymethylene with molecular sieves as catalysts". Journal of Fuel Chemistry and Technology. 39 (12): 918–923. doi:10.1016/S1872-5813(12)60003-6.
- ^ Pellegrini, L., Marchionna, M., Patrini, R., and Florio, S. (2013). "Emission Performance of Neat and Blended Polyoxymethylene Dimethyl Ethers in an Old Light-Duty Diesel Car". SAE Technical Paper Series. Vol. 1. doi:10.4271/2013-01-1035.
{{cite book}}:|work=ignored (help)CS1 maint: multiple names: authors list (link)
