Talk:Methoxyethane
| This article is rated Stub-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
what determines thename[edit]
why is it called methoxyethane and not ethoxymethane —Preceding unsigned comment added by Giza182 (talk • contribs) 18:57, 9 September 2010 (UTC)
- Because the International Union of Pure and Applied Chemistry (IUPAC) have decreed it to be so. DieSwartzPunkt (talk) 16:50, 11 June 2012 (UTC)
Missing atoms[edit]
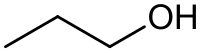
The diagram at the top of the info box seems to missing a carbon atom along with a couple of hydrogen.
Also, shouldn't the chemical formula reflect the molecular makeup as C3H8O could be one of 3 different substances (two not even having the same chemical properties). Shouldn't the formula be something like CH3.C2H5.O to distinguish it from (say) C3H7.OH (Propanol)?
And finally, as its boiling point is only 11 Celcius, surely it is a gas and not a liquid (at NTP). 109.157.160.61 (talk) 17:50, 9 June 2012 (UTC)
- The diagram isn't missing anything. In a skeletal form like that shown, it is automatically assumed that where the lines join or end that there is a carbon atom with as many hydrogen atoms as necessary to make up the valency of 4. In fact the CH3 at the 2 ends could have been left blank. Only deviations from carbon and hydrogen are usually shown. See this one for Propyl alcohol.
- Or even this one for Pentane.
- I have to say that I am also used to seeing the chemical formulae arranged in groups. Just looking at my bottle of isopropyl alcohol it has the fomula as (CH3)2CHOH rather than C3H8O. A quick google at some external sites states that Methoxyethane is indeed a gas at normal temperature and pressure. But since I am not an expert in organic chemistry, I would prefer to leave any changes to someone more qualified. If that is you then be bold and change them. Someone will soon point out your error if you are wrong. DieSwartzPunkt (talk) 16:48, 11 June 2012 (UTC)
I've updated the chemical infobox with references - as you found, methoxyethane is a gas at room temperature. It's very useful to keep the molecular formula as an empirical formula - people will want to know how many atoms of each element there are without having to add up separate parts. The structure is already given in the chembox, so we're not providing any more information with a semi-structural formula. The use of lines and omission of hydrogens is called a skeletal formula. --Ben (talk) 19:51, 11 June 2012 (UTC)