Talk:Phosphorus trioxide
| This article is rated Start-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Name[edit]
Why isn't this compound called tetraphosphorus hexoxide?? Georgia guy 23:14, 17 April 2007 (UTC)
HAPPY
P4O6 as a ligand[edit]
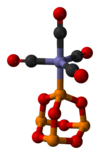
I've made an image (based on xray data from Acta Cryst. (1996). C52, 2650-2652) of a complex with P4O6 as a ligand. At the moment, the article seems a bit short to include the image and related discussion, since there are probably several other more significant aspects of phosphorus trioxide's chemistry that should be expanded upon first.
I'll leave the image here for now and I'll consider extending the article generally when I have time.
Ben (talk) 11:48, 18 January 2009 (UTC)
- You should include the image in the article. A variety of such complexes are known but I dont think that they ever did anything noteoworthy. Their existence reminds readers that the cage has phosphite-like character. I dont know what even P4O6 is good for really.
- On a related topic the current article states
- "It reacts with cold water to form phosphorous acid.
- P4O6(s) + 6 H2O → 4 H3PO3
- "It reacts with cold water to form phosphorous acid.
- It reacts with hot water vigorously to form flammable phosphine gas ( PH3 ).
- P4O6(s) + 6 H2O(l) → PH3(g) + 3 H3PO4"
- which does not make a lot of sense unless phosphorous acid is an intermediate in the disproportionation process. Greenwood and Earnshaw would have this.--Smokefoot (talk) 14:31, 18 January 2009 (UTC)
I'll read what Greenwood and Earnshaw have to say on the matter, plus my other undergraduate (and maybe school) textbooks, and add what I can from a student's perspective. Then you pros can correct what I've done and add any advanced bits you think would be beneficial.
Cheers
Ben (talk) 14:41, 18 January 2009 (UTC)
- G&E has the cold water reaction (suggesting there is a different reaction in hot water), and a few (old) papers and books I found online support this. Can't access many though. I'll have a look at Hollemann and Wiberg.
I've added more about phosphorus trioxide as a ligand. I haven't had a chance to write this bit yet, but the iron tetracarbonyl complex can be prepared by a photochemical reaction of P4O6 and Fe(CO)5: Inorg. Chem., 1975, 14 (10), pp 2438–2440.
Ben (talk) 00:05, 9 February 2009 (UTC)
Acidity[edit]
The article lists a pKa of 9.2 without any reference -- presumably this is copied from arsenic trioxide. Maybe we should put a link to phosphorous acid there instead. Zhieaanm (talk) 02:33, 22 January 2011 (UTC)