User:A1abbas/sandbox
This is the user sandbox for user A1abbas. It was created on June 7, 2017.
Biosynthesis -
The biosynthesis of thujone is similar to the synthesis of other monoterpenes and begins with the formation of geranyl diphosphate (GPP) from dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP), catalyzed by the enzyme geranyl diphosphate synthase [1]. Quantitative 13CNMR spectroscopic analysis has demonstrated that the isoprene units used to form thujone in plants are derived from the methylerythritol phosphate pathway (MEP) [2].
The reactions that generate the thujane skeleton in sabinene from GPP are mediated by the enzyme sabinene synthase which has GPP as its substrate[1]. GPP (1) first isomerizes to linalyl diphosphate (LPP) (2) and neryl diphosphate (NPP) (3). LPP preferentially forms a delocalized allylic cation-diphosphate (4). The ion-pair intermediate then cyclizes in an electrophilic addition to yield the α-terpinyl tertiary cation (5)[1].
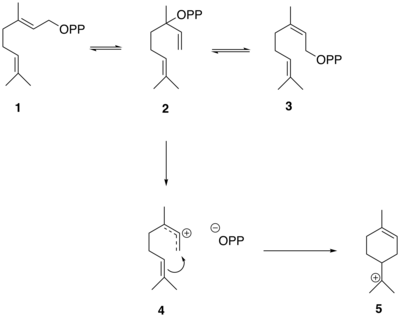
The α-terpinyl cation (5) then undergoes a 1,2 hydride shift via a Wagner–Meerwein rearrangement, leading to the formation of the terpinen-4-yl cation (6). This cation undergoes a second cyclization to form the thujyl cation intermediate (7) before loss of a proton to form the thujone precursor, (+)-sabinene (8).
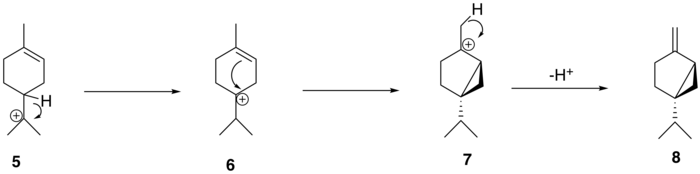
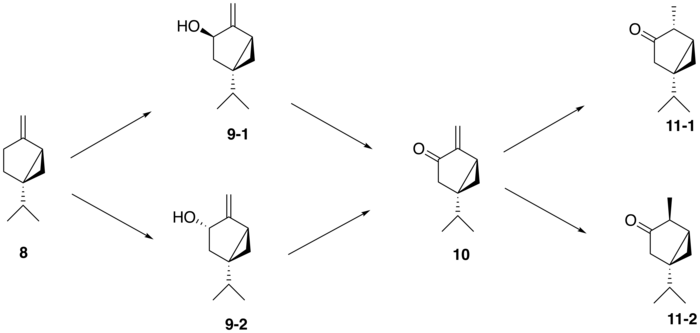
From (+)-sabinene (8), the proposed biosynthetic route to generate thujone follows a three-step pathway: (+)-sabinene is first oxidized to an isomer of (+)-sabinol (9-1,2) by a cytochrome P450 enzyme, followed by conversion to (+)-sabinone (10) via a dehydrogenase. Finally, a reductase mediates the conversion to α-thujone (11-1) and β-thujone (11-2)[3]. The isomerism of the (+)-sabinol intermediate varies among thujone-producing plants; for instance, in the western redcedar (Thuja plicata), thujone is derived exclusively from the (+)-trans-sabinol intermediate (9-1) whereas in the common garden sage (Salvia officinalis), thujone is formed from the (+)-cis-sabinol intermediate (9-2)[4].
- ^ a b c Dewick, Paul M (2009). Medicinal natural products: a biosynthetic approach (3rd ed.). John Wiley & Sons Ltd. p. 195-197. ISBN 978-0-470-74167-2.
- ^ Umlauf, Dirk; Zapp, Josef (September 2004). "Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae)". Phytochemistry. 65 (17): 2463–2470. doi:10.1016/j.phytochem.2004.08.019. Retrieved June 7, 2017.
{{cite journal}}: Check|url=value (help) - ^ Foster, Adam J.; Hall, Dawn E. (Apr 2013). "Identification of Genes in Thuja plicata Foliar Terpenoid Defenses". Plant Physiology. 161 (4): 1993–2004. doi:10.1104/pp.112.206383. Retrieved June 7, 2017.
- ^ Gesell, Andreas; Blaukopf, Markus (May 2015). "The Gymnosperm Cytochrome P450 CYP750B1 Catalyzes Stereospecific Monoterpene Hydroxylation of (+)-Sabinene in Thujone Biosynthesis in Western Redcedar". Plant Physiology. 168 (1): 94–106. doi:10.1104/pp.15.00315. Retrieved June 7, 2017.

