User:Anicm1/sandbox
 | This is a user sandbox of Anicm1. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Awareness in patients with disorders of consciousness
There are three disorders of consciousness (DoC): coma, vegetative state (VS), and minimally conscious state (MCS). Amantadine has been shown to increase the rate of emergence from a MCS, defined by consistent demonstration of interactive communication and functional objective use. In traumatic brain injury patients in the intensive care unit, amantadine has also been shown in various randomized control trials to increase the rate of functional recovery and arousal, particularly in the time period immediately following an injury.[1] There are also reports of significantly improved consciousness in patients treated for non-traumatic cases of DoC, such as in the case of a subarachnoid hemorrhage, cerebral hemorrhage, and hypoxic encephalopathy.[2] In 2018 the American Academy of Neurology (AAN) updated treatment guidelines on the use of amantadine for patients with prolonged DoC, recommending the use of amantadine (100-200 mg bid) for adults with DoC 4-16 weeks post injury to support early functional recovery and reduce disability.[3]
Neuroplasticity and overall functional recovery in patients with brain injury
In various studies, amantadine and memantine have been shown to accelerate the rate of recovery from a brain injury. The time-limited window following a brain injury is characterized by neuroplasticity, or the capacity of neurons in the brain to adapt and compensate after injury. Thus, physiatrists will often start patients on amantadine as soon as impairments are recognized. Yet, there are also case reports showing improved functional recovery with amantadine treatment occurring years after the initial brain injury.[4] There is insufficient evidence to determine if the functional gains are a result of effects through the dopamine or norepinephrine pathways. Some patients may benefit from direct dopamine stimulation with amantadine, while others may benefit more from other stimulants that act more on the norepinephrine pathway, such as methylphenidate.[4] It is unclear if treatment with amantadine improves long-term outcomes or simply accelerates recovery.[5] Nonetheless, amantadine-induced acceleration of recovery reduces the burden of disability, lessens health care costs, and minimizes psychosocial stressors in patients and caregivers
Fatigue in multiple sclerosis[edit]
Amantadine, along with modafinil and methylphenidate, are the most commonly used medications for the treatment of multiple sclerosis (MS) related fatigue in clinical practice. A 2007 Cochrane literature review concluded that there was no overall evidence supporting the use of amantadine in treating fatigue in patients with MS.[6] A follow up 2012 Cochrane review stated that there may be some amantadine-induced improvement in fatigue in some people with MS.[7] Despite multiple control trials that have also demonstrated improvements in subjective and objective ratings of fatigue, there is no final conclusion regarding its effectiveness.[8]
Consensus guidelines from the German Multiple Sclerosis Society (GMSS) in 2006 state that amantadine produces moderate improvement in subjective fatigue, problem solving, memory, and concentration. Thus, GMSS guidelines strongly recommend the use of amantadine in MS-related fatigue.[9]
Contraindications[edit]
Amantadine is contraindicated in persons with end stage renal disease. The drug is renally cleared.
Amantadine may have anticholinergic side effects. Thus, patients with an enlarged prostate or glaucoma should use with caution. [10]
Live attenuated vaccines are contraindicated while taking amantadine. It is possible that amantadine will inhibit viral replication and reduce the efficacy of administered vaccines. The U.S. Food and Drug Administration (FDA) recommends avoiding amantadine for two weeks prior to vaccine administration and 48 hours afterwards.
Adverse effects[edit]
Amantadine is generally well tolerated and has a mild side-effect profile.
Neurological[edit]
Side effects include drowsiness (especially while driving), light headedness, falls, and dizziness. Patients on Amantadine should avoid combination with other CNS depressing agents, such as alcohol. Excessive alcohol usage may increase the potential for CNS effects such as dizziness, confusion, lightheadedness and orthostatic hypotension. [10]
Rare severe adverse effects include neuroleptic malignant syndrome, psychosis, depression, suicidal ideation, and convulsions. It has also been associated with inhibited actions (gambling, sexual activity, spending, other addictions) and diminished control over compulsions.
Cardiovascular[edit]
Amantadine may cause orthostatic hypotension, syncope, and peripheral edema.
Gastrointestinal[edit]
Amantadine has also been associated with dry mouth and constipation.
Skin[edit]
Rare cases of skin rashes, such as Stevens–Johnson syndrome and livedo reticularis have also been reported in patients treated with Amantadine.
Pregnancy and lactation[edit]
Amantadine is a FDA category C for pregnancy. Teratogenic effects have been observed in humans (case reports) and animal reproduction studies . Amantadine may also be present in breast milk and alter breast milk production or excretion. The decision to breastfeed during therapy should consider the risk of infant exposure, the benefits of breastfeeding, and the benefits of the drug to the mother.[10]
Parkinson's disease[edit]
The mechanism of its antiparkinsonian effect is poorly understood.[11] Amantadine appears to be a weak non-competitive antagonist of the NMDA-type glutamate receptor, increases dopamine release, and blocks dopamine reuptake.[12][13][14] Amantadine probably does not inhibit monoamine oxidase (MAO) enzyme.[15] Moreover, the drug has many effects on the pre-synaptic membrane, including enhancing the release of dopamine and inhibiting its reuptake. Post-synaptically, amantadine acts directly on the dopamine receptor and upregulates D2 receptors. The anti-glutamatergic properties of amantadine can reduce the severity of levodopa-induced dyskinesia. It appears to be an anticholinergic (specifically at alpha-7 nicotinic receptors) like the similar pharmaceutical memantine.[1]
In 2004, it was discovered that amantadine and memantine bind to and act as agonists of the σ1 receptor (Ki = 7.44 μM and 2.60 μM, respectively), and that activation of the σ1 receptor is involved in the dopaminergic effects of amantadine at therapeutically relevant concentrations.[16] These findings may also extend to the other adamantanes such as adapromine, rimantadine, and bromantane, and could explain the psychostimulant-like effects of this family of compounds.[16]
Brain injury and disorders of consciousness[edit]
The mechanism of amantadine's use in brain injury recovery is related to its focal effects on both dopamine and NMDA receptors, as well as its anti-inflammatory properties. NMDA receptors are excitatory glutamate receptors. The receptors are composed of ion channels that contain binding sites for antagonists such as amantadine and memantine. The binding of glutamate to these receptors causes excitation, which can be toxic in excess. Thus, antagonism of the NMDA receptor reduces toxicity, which plays a key role in brain development and synaptic plasticity.[17]
Amantadine and memantine also have mild anti-inflammatory properties. There is evidence that amantadine reduces the release of pro-inflammatory factors from microglia and inflammatory tumor necrosis factor from the hippocampus.[1]
Another proposed mechanism for increasing clinical arousal is through increased dopamine release. This concept is based on the enhanced dopamine targeting of the of three major dopaminergic pathways in the brain involved in increasing awareness in patients with brain injury and DoC: the mesolimbic, mesocortical, and nigrostriatal pathways.[1] The mesolimbic pathway is associated with motivations, memory, and learning. The mesocortical pathway is associated with motivation, activation, planning, and cortical tone and attention. The nigrostriatal pathway is involved in initiation and velocity of movement.
Influenza[edit]

The mechanisms for amantadine's antiviral and antiparkinsonian effects are unrelated.[19][20] Amantadine targets the influenza A M2 ion channel protein. The M2 protein's function is to allow the intracellular virus to replicate (M2 also functions as a proton channel for hydrogen ions to cross into the vesicle), and exocytose newly formed viral proteins to the extracellular space (viral shedding). By blocking the M2 channel, the virus is unable to replicate because of impaired replication, protein synthesis, and exocytosis.[21][22]
Amantadine and rimantadine function in a mechanistically identical fashion, entering the barrel of the tetrameric M2 channel and blocking pore function—i.e., proton translocation.[23]
Resistance to the drug class is a consequence of mutations to the pore-lining residues of the channel, preventing both amantadine and rimantadine from inhibiting the channel in their usual way.[citation needed]
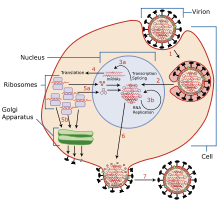
Influenza B strains possess a structurally distinct M2 channel with channel-facing side chains that fully obstruct the channel vis-à-vis binding of adamantine-class channel inhibitors, while still allowing proton flow and channel function to occur; this constriction in the channels is responsible for the ineffectiveness of this drug and rimantadine towards all circulating Influenza B strains.[citation needed]
Interactions[edit]
Amantadine may affect the central nervous system due to dopaminergic and anticholinergic properties. The mechanisms of action are not fully known. Because of the CNS effects, caution is required when prescribing additional CNS stimulants or anticholinergic drugs.[24] Thus, concurrent use of alcohol with Amantadine is not recommended due to enhanced CNS depressant effects.[25] In addition, anti-dopaminergic drugs such as metoclopramide and typical anti-psychotics should be avoided.[26][27] These interactions are likely related to opposing dopaminergic mechanisms of action, which inhibits amantadine's anti-Parkinson effects.
Certain drugs increase the chances of déjà vu occurring in the user, resulting in a strong sensation that an event or experience currently being experienced has already been experienced in the past. Some pharmaceutical drugs, when taken together, have also been implicated in the cause of déjà vu. Taiminen and Jääskeläinen (2001)[28] reported the case of an otherwise healthy male who started experiencing intense and recurrent sensations of déjà vu upon taking the drugs amantadine and phenylpropanolamine together to relieve flu symptoms. He found the experience so interesting that he completed the full course of his treatment and reported it to the psychologists to write up as a case study. Because of the dopaminergic action of the drugs and previous findings from electrode stimulation of the brain (e.g. Bancaud, Brunet-Bourgin, Chauvel, & Halgren, 1994),[29] Taiminen and Jääskeläinen speculate that déjà vu occurs as a result of hyperdopaminergic action in the mesial temporal areas of the brain.
History[edit]
Antiviral properties were first reported in 1963 at the University of Illinois Hospital in Chicago. In this amantadine trial study volunteer college students were exposed to a viral challenge. The group that received amantadine (100 milligrams 18 hours before viral challenge) had less Asia influenza infections than the placebo group.[30] Amantadine received approval for the treatment of influenza virus A[31][32][33][34] in adults in 1976.[30] It was first used in the FRG in 1966. Amantadine was approved by the U.S. Food and Drug Administration (FDA) in October 1968, as a prophylactic agent against Asian (H2N2) influenza and received approval for prophylactic use for influence A in 1976.[30][35][36]
An incidental finding in 1969 prompted investigations about amantadine's effectiveness for treating symptoms of Parkinson's disease.[30] A woman with Parkinson's disease was prescribed amantadine to treat her influenza infection and reported her cogwheel rigidity and tremors improved. She also reported that her symptoms worsened after she finished the course of amantadine.[30] The published case report was not initially corroborated by any other instances by the medical literature or manufacturer data. A team of researchers looked at a group of ten patients with Parkinson's disease and gave them amantadine. Seven of the ten patients showed improvement, which was convincing evidence for the need of a clinical trial. The 1969 trial (lead author Robert S. Schwab, MD) included 163 patients with Parkinson's disease and 66% experienced subjective or objective reduction of symptoms with a maximum daily dose of 200 mg.[30][37] Additional studies from Schwab's team followed patients for greater lengths of time and in different combinations of neurological drugs.[38] It was found to be a safe drug that could be used over long periods of time with few side effects, as monotherapy or in combination with L-dopa or anti-cholinergic drugs.[30] By April 1973, the U.S. Food and Drug Administration (FDA) approved amantadine for use in the treatment of Parkinson's disease.[39][30]
In 2017, the U.S. Food and Drug Administration (FDA) approved the use of amantadine in an extended release formulation developed by Adamas Pharma for the treatment of dyskinesia, an adverse effect of levodopa in patients with Parkinson's disease.[40][41]
Veterinary misuse[edit]
In 2005, Chinese poultry farmers were reported to have used amantadine to protect birds against avian influenza.[42] In Western countries and according to international livestock regulations, amantadine is approved only for use in humans. Chickens in China have received an estimated 2.6 billion doses of amantadine.[42] Avian flu (H5N1) strains in China and southeast Asia are now resistant to amantadine, although strains circulating elsewhere still seem to be sensitive. If amantadine-resistant strains of the virus spread, the drugs of choice in an avian flu outbreak will probably be restricted to neuraminidase inhibitors oseltamivir and zanamivir which block the action of viral neuraminidase enzyme on the surface of influenza virus particles.[43] However, there is an increasing incidence of oseltamivir resistance in circulating influenza strains (e.g., H1N1), highlighting the serious need for the development of new anti-influenza therapies.[44]
In September 2015, the U.S. Food and Drug Administration announced the recall of Dingo Chip Twists "Chicken in the Middle" dog treats because the product has the potential to be contaminated with amantadine.[45]
Resistance[edit]
During the 1980 influenza A epidemic, the first amantadine-resistance influenza viruses were reported. The frequency of amantadine resistance among influenza A (H3N2) viruses from 1991 and 1995 was as low as 0.8%. In 2004 the resistance frequency increased to 12.3%. A year later resistance increase significantly to 96%, 72%, and 14.5% in China, South Korea, and the United States, respectively. By 2006, 90.6% of H3N2 strains and 15.6% of H1N1 were amantadine-resistant. A majority of the amantadine-resistant H3N2 isolates (98.2%) were found to contain an S31N mutation in the M2 transmembrane domain that confers resistance to amantadine.[46] Currently, adamantane resistance is high among circulating influenza A viruses. Thus, they are no longer recommended for treatment of influenza A.[47]
Pharmacokinetics[edit]
Amantadine is well absorbed orally from the GI tract. The onset of action is usually within 48 hours when used for parkinsonian syndromes, including dyskinesia. Peak plasma concentrations are related to dose for doses up to 200 mg/day. Doses above 200 mg/day may result in a greater than proportional increase in peak plasma concentration. As plasma concentrations of amantadine increase, there is a greater risk for toxicity.[48][49]
Compared with healthy adults, the plasma clearance of amantadine is significantly reduced and plasma concentrations are increased in healthy elderly individuals age 60 and older and adult patients with renal insufficiency. The elimination half-life increases 2-3 fold or greater when creatinine clearance is less than 40 mL/min/1.73 m. Half-life elimination averages 8 days in patients with end-stage renal disease. Amantadine is only minimally removed by hemodialysis.[49][50]
Amantadine is metabolized to a small extent (5-15%) by acetylation. It is mainly excreted (90%) unchanged in urine by glomerular filtration and tubular secretion.[48]
Amantadine has a mild side effect profile. Common neurological side effects include drowsiness, light headedness, dizziness, and confusion.[10] Due to its effects on the central nervous system, it should not be combined with additional CNS stimulants or anticholinergic drugs. Amantadine is contraindicated in persons with end stage renal disease, given that the drug is renally cleared.[35] It should also be taken with caution in those with enlarged prostates or glaucoma, due to its anticholinergic effects.[20] Live attenuated vaccines are contraindicated while taking amantadine, as it may limit the efficacy of the administered vaccine.[35]
The history of amantadine's discovery and use for treating influenza A. After antiviral properties were reported in 1963, amantadine recieved approval for prophylactic use for influenza virus A in 1976. However, amantadine-resistant influenza viruses were first reported during the 1980 influenza A epidemic and resistance frequency continued to rise into the early 2000s. Currently, amantadine is no longer recommended for the treatment of influenza A due to a high level of amantadine resistance among circulating influenza A viruses.
- ^ a b c d Ma, Heather M.; Zafonte, Ross D. (2020-02-20). "Amantadine and memantine: a comprehensive review for acquired brain injury". Brain Injury. 34 (3): 299–315. doi:10.1080/02699052.2020.1723697. ISSN 0269-9052.
- ^ Gao, Yu; Ma, Linlin; Liang, Fang; Zhang, Yi; Yang, Lin; Liu, Xuehua; Yang, Jing (2 July 2020). "The use of amantadine in patients with unresponsive wakefulness syndrome after severe cerebral hemorrhage". Brain Injury. 34 (8): 1084–1088. doi:10.1080/02699052.2020.1780315.
- ^ Giacino, Joseph T.; Katz, Douglas I.; Schiff, Nicholas D.; Whyte, John; Ashman, Eric J.; Ashwal, Stephen; Barbano, Richard; Hammond, Flora M.; Laureys, Steven; Ling, Geoffrey S.F.; Nakase-Richardson, Risa; Seel, Ronald T.; Yablon, Stuart; Getchius, Thomas S.D.; Gronseth, Gary S.; Armstrong, Melissa J. (4 September 2018). "Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research". Neurology. 91 (10): 450–460. doi:10.1212/WNL.0000000000005926.
- ^ a b Ma, Heather M.; Zafonte, Ross D. (23 February 2020). "Amantadine and memantine: a comprehensive review for acquired brain injury". Brain Injury. 34 (3): 299–315. doi:10.1080/02699052.2020.1723697.
- ^ Giacino, Joseph T.; Whyte, John; Bagiella, Emilia; Kalmar, Kathleen; Childs, Nancy; Khademi, Allen; Eifert, Bernd; Long, David; Katz, Douglas I.; Cho, Sooja; Yablon, Stuart A.; Luther, Marianne; Hammond, Flora M.; Nordenbo, Annette; Novak, Paul; Mercer, Walt; Maurer-Karattup, Petra; Sherer, Mark (March 2012). "Placebo-Controlled Trial of Amantadine for Severe Traumatic Brain Injury". New England Journal of Medicine. 366 (9): 819–826. doi:DOI: 10.1056/NEJMoa1102609.
{{cite journal}}: Check|doi=value (help) - ^ Pucci, Eugenio; Brañas Tato, Pedro; D'Amico, Roberto; Giuliani, Giorgio; Solari, Alessandra; Taus, Cristiana (2007-01-24). Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group (ed.). "Amantadine for fatigue in multiple sclerosis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD002818.pub2. PMC 6991937. PMID 17253480.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Payne, Cathy; Martin, Suzanne; Wiffen, Philip J (2010-03-17), "Interventions for fatigue and weight loss in adults with advanced progressive illness", Cochrane Database of Systematic Reviews, Chichester, UK: John Wiley & Sons, Ltd, retrieved 2020-11-02
- ^ Generali, Joyce A.; Cada, Dennis J. (2014-09). "Amantadine: Multiple Sclerosis–Related Fatigue". Hospital Pharmacy. 49 (8): 710–712. doi:10.1310/hpj4908-710. ISSN 0018-5787. PMC 4252198. PMID 25477595.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Henze, T.; Rieckmann, P.; Toyka, K.V. (2006). "Symptomatic Treatment of Multiple Sclerosis". European Neurology. 56 (2): 78–105. doi:10.1159/000095699. ISSN 0014-3022.
- ^ a b c d Chang, Carol; Ramphul, Kamleshun (2020), "Amantadine", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763128, retrieved 2020-11-02
- ^ Chang, Carol; Ramphul, Kamleshun (2020), "Amantadine", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763128, retrieved 2020-11-07
- ^ Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. p. 3962. ISBN 978-3-85200-181-4.
- ^ Blanpied TA, Clarke RJ, Johnson JW (March 2005). "Amantadine inhibits NMDA receptors by accelerating channel closure during channel block". The Journal of Neuroscience. 25 (13): 3312–22. doi:10.1523/JNEUROSCI.4262-04.2005. PMC 6724906. PMID 15800186.
- ^ Deleu, Dirk; Northway, Margaret G.; Hanssens, Yolande (2002). "Clinical Pharmacokinetic and Pharmacodynamic Properties of Drugs Used in the Treatment of Parkinson??s Disease:". Clinical Pharmacokinetics. 41 (4): 261–309. doi:10.2165/00003088-200241040-00003. ISSN 0312-5963.
- ^ Strömberg U, Svensson TH (November 1971). "Further studies on the mode of action of amantadine". Acta Pharmacologica Et Toxicologica. 30 (3): 161–71. doi:10.1111/j.1600-0773.1971.tb00646.x. PMID 5171936.
- ^ a b Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E (April 2004). "Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine". The European Journal of Neuroscience. 19 (8): 2212–20. doi:10.1111/j.0953-816X.2004.03297.x. PMID 15090047. S2CID 19479968.
- ^ Kalia, Lorraine V; Kalia, Suneil K; Salter, Michael W (August 2008). "NMDA receptors in clinical neurology: excitatory times ahead". The Lancet Neurology. 7 (8): 742–755. doi:10.1016/S1474-4422(08)70165-0. PMC 3589564. PMID 18635022.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Thomaston JL, Alfonso-Prieto M, Woldeyes RA, Fraser JS, Klein ML, Fiorin G, DeGrado WF (November 2015). "High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction". Proceedings of the National Academy of Sciences of the United States of America. 112 (46): 14260–5. Bibcode:2015PNAS..11214260T. doi:10.1073/pnas.1518493112. PMC 4655559. PMID 26578770.
- ^ "Australian Product Information – Symmetrel (Amantadine Hydrochloride) Capsules". Australian Department of Health Therapeutic Goods Administration. Retrieved 13 July 2019.
- ^ a b "Symmetrel (Amantadine Hydrochloride, USP) fact sheet" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 28 July 2019.
- ^ PubChem. "Amantadine". pubchem.ncbi.nlm.nih.gov. Retrieved 29 July 2019.
- ^ Staničová, J.; Miškovský, P.; Šutiak, V. (2001-01-01). "Amantadine: an antiviral and antiparkinsonian agent". Veterinární Medicína. 46 (No. 9–10): 275–277. doi:10.17221/7884-vetmed. ISSN 0375-8427.
{{cite journal}}:|issue=has extra text (help) - ^ Golan DE, Armstrong EJ, Armstrong AW (2017). Principles of pharmacology: the pathophysiologic basis of drug therapy (4th ed.). Philadelphia: Wolters Kluwer. pp. 142, 199, 205t, 224t, 608, 698–700. ISBN 9781451191004. OCLC 914593652.
- ^ "Symmetrel (Amantadine Hydrochloride, USP) fact sheet" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 28 July 2019.
- ^ Gocovri (amantadine) extended-release capsules [prescribing information]. Emeryville, CA: Adamas Pharma, LLC; August 2017
- ^ Reglan (metoclopramide) [prescribing information]. Baudette, MN: ANI Pharmaceuticals Inc; August 2017
- ^ Tarsy, D; Parkes, J D; Marsden, C D (1975-04-01). "Metoclopramide and pimozide in Parkinson's disease and levodopa-induced dyskinesias". Journal of Neurology, Neurosurgery & Psychiatry. 38 (4): 331–335. doi:10.1136/jnnp.38.4.331. ISSN 0022-3050. PMC 491929. PMID 1095689.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Taiminen T, Jääskeläinen SK (September 2001). "Intense and recurrent déjà vu experiences related to amantadine and phenylpropanolamine in a healthy male". Journal of Clinical Neuroscience. 8 (5): 460–2. doi:10.1054/jocn.2000.0810. PMID 11535020. S2CID 6733989.
- ^ Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E (February 1994). "Anatomical origin of déjà vu and vivid 'memories' in human temporal lobe epilepsy". Brain. 117 ( Pt 1) (1): 71–90. doi:10.1093/brain/117.1.71. PMID 8149215.
- ^ a b c d e f g h Hubsher G, Haider M, Okun MS (April 2012). "Amantadine: the journey from fighting flu to treating Parkinson disease". Neurology. 78 (14): 1096–9. doi:10.1212/WNL.0b013e31824e8f0d. PMID 22474298. S2CID 21515610.
- ^ Hounshell DA, Kenly Smith J (1988). Science and Corporate Strategy: Du Pont R&D, 1902–1980. Cambridge University Press. p. 469. ISBN 978-0521327671.
- ^ "Sales of flu drug by du Pont unit a 'disappointment'". The New York Times. Wilmington, Delaware. 5 October 1982. Retrieved 19 May 2008.
- ^ Maugh TH (November 1979). "Panel urges wide use of antiviral drug". Science. 206 (4422): 1058–60. Bibcode:1979Sci...206.1058M. doi:10.1126/science.386515. PMID 386515.
- ^ Maugh TH (April 1976). "Amantadine: an alternative for prevention of influenza". Science. 192 (4235): 130–1. doi:10.1126/science.192.4235.130. PMID 17792438.
- ^ a b c "Gocovri- amantadine capsule, coated pellets". DailyMed. 26 December 2019. Retrieved 22 January 2020.
- ^ "International Review of Neurobiology", International Review of Neurobiology, Elsevier, pp. i–iii, 2011, ISBN 978-0-12-387003-2, retrieved 2020-11-11
- ^ Schwab, Robert S. (19 May 1969). "Amantadine in the Treatment of Parkinson's Disease". JAMA: The Journal of the American Medical Association. 208 (7): 1168. doi:10.1001/jama.1969.03160070046011. ISSN 0098-7484.
- ^ Schwab RS, Poskanzer DC, England AC, Young RR (November 1972). "Amantadine in Parkinson's disease. Review of more than two years' experience". JAMA. 222 (7): 792–5. doi:10.1001/jama.222.7.792. PMID 4677928.
- ^ "Symmetrel (Amantadine Hydrochloride, USP) fact sheet" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 28 July 2019.
- ^ Bastings E. "NDA 208944 Approval Letter" (PDF).
- ^ "Drug Approval Package: Gocovri (amantadine extended-release)". U.S. Food and Drug Administration (FDA). 29 June 2018. Retrieved 22 January 2020.
- ^ a b Sipress A (18 June 2005). "Bird Flu Drug Rendered Useless". The Washington Post. Retrieved 2 August 2007.
- ^ Kumar B, Asha K, Khanna M, Ronsard L, Meseko CA, Sanicas M (April 2018). "The emerging influenza virus threat: status and new prospects for its therapy and control". Archives of Virology. 163 (4): 831–844. doi:10.1007/s00705-018-3708-y. PMC 7087104. PMID 29322273.
- ^ Aoki FY, Boivin G, Roberts N (2007). "Influenza virus susceptibility and resistance to oseltamivir". Antiviral Therapy. 12 (4 Pt B): 603–16. PMID 17944268.
- ^ "Enforcement Report – Week of September 23, 2015". FDA.gov. U.S. Food and Drug Administration (FDA).
- ^ Kumar, Binod; Asha, Kumari; Khanna, Madhu; Ronsard, Larance; Meseko, Clement Adebajo; Sanicas, Melvin (2018-04-01). "The emerging influenza virus threat: status and new prospects for its therapy and control". Archives of Virology. 163 (4): 831–844. doi:10.1007/s00705-018-3708-y. ISSN 1432-8798. PMC 7087104. PMID 29322273.
{{cite journal}}: CS1 maint: PMC format (link) - ^ "Antiviral Drug Resistance among Influenza Viruses | CDC". www.cdc.gov. 2019-04-17. Retrieved 2020-11-11.
- ^ a b "Amantadine Hydrochloride Monograph for Professionals". Drugs.com. Retrieved 2020-11-16.
- ^ a b "Amantadine - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 2020-11-16.
- ^ Deleu, Dirk; Northway, Margaret G.; Hanssens, Yolande (2002). "Clinical Pharmacokinetic and Pharmacodynamic Properties of Drugs Used in the Treatment of Parkinson??s Disease". Clinical Pharmacokinetics. 41 (4): 261–309. doi:10.2165/00003088-200241040-00003. ISSN 0312-5963.

