User:BQUB16-Mmiranda/sandbox/Asparagine peptide lyases
| This is not a Wikipedia article: It is an individual user's work-in-progress page, and may be incomplete and/or unreliable. For guidance on developing this draft, see Wikipedia:So you made a userspace draft. Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
Asparagine peptide lyases new article content ...
Asparagine peptide lyases are one of the seven groups in which proteases, also termed proteolytic enzymes, peptidases or proteinases, are classified according to their catalytic residue. [1]
The existence of this seventh catalytic type of proteases, in which the peptide bond cleavage occurs by self-processing instead of hydrolysis, was demonstrated with the discovery of the crystal structure of the self-cleaving precursor of the Tsh autotransporter from E. coli. [2]
Asparagine peptide lyases catalytic mechanism involves an asparagine residue as nucleophile to perform a nucleophilic elimination reaction, rather than hydrolysis, to catalyse the breaking of peptide bonds. [3]
Synthesis[edit]
The inclusion of asparagine peptide lyases as proteolytic enzymes might be seen as controversial because they perform a self-cleaving process and are destroyed by the enzymatic activity. It has been demonstrated that self-processing is the action of a proteolytic enzyme, notwithstanding the enzyme is not recoverable from the reaction. [4]
Asparagine peptide lyases are synthesized as precursors or propeptides, which cleave themselves by an autoproteolytic reaction, always involving an asparagine residue. [5]
BQUB16-Mmiranda (talk) 09:50, 22 October 2016 (UTC)
Active site and catalytic mechanism[edit]
All the proteolytic activity of the asparagine peptide lyases is only self-cleavages, then no further peptidase activity occurs. [6][7]
The main residue of the active site is the asparagine and there are other residues involved in the catalytic mechanism, which are different between the different families of asparagine peptide lyases. [8][9][10]
The cleavage mechanism consists in the cyclization of the asparagine, assisted by other active site residues. In certain conditions, the asparagine cyclic structure nucleophilically attacks its C-terminal peptide bond to the main chain forming a new bond to create a stable succinimide, cleaving itself from the main chain and consequently releasing the two halves of the product. [11][12]
BQUB16-Mmiranda (talk) 09:50, 22 October 2016 (UTC)
Inhibition[edit]
No inhibitors are known.
Classification[edit]
The MEROPS protease database includes the following ten families of asparagine peptide lyases, which are included in 6 different clans of proteases.
Proteolytic enzymes are classified into families based on sequence similarity. Each family includes proteolytic enzymes with homologous sequences and common catalytic type. Clans are groups of proteolytic enzymes families with related structures, where catalytic type is not conserved.
| Header text | Header text | Header text | Header text | Header text | Header text |
|---|---|---|---|---|---|
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
| Example | Example | Example | Example | Example | Example |
Distribution and types[edit]
The ten different families of asparagine peptide lyases are distributed in three different types: - Viral coat proteins - Autotransporter proteins - Intein-containing proteins There are five families of viral coat proteins (N1, N2, N8, N7 and N5), two families of autotransporter proteins (N6 and N4) and three families of intein-containing proteins (N9, N10 and N11).
Viral coat proteins[edit]
Autotransporter proteins[edit]
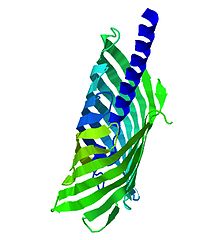
Autotransporter proteins are outer membrane or secreted proteins found in a broad variety of Gram-negative bacteria. These proteins contain three structural motifs: a signal sequence, a passenger domain located at the N-terminal, and an autotransporter or translocator domain located at the C-terminal, forming a -barrel structure. These structures promote the protein self-transport. Autotransporter proteins are usually related to virulence functions. This fact, their interaction with host cells and the broad occurrence of autotransporter encoding genes, bring up the possibility to represent therapeutic targets for the design of vaccines against Gram-negative pathogens. [14]
Two of the families in which the MEROPS database classifies asparagine peptide lyases are autotransporter proteins, families N4 and N6. [15]
Family N4 includes secreted virulence factors, or autotransporters, from enterobacteria. Their only proteolytic activity is releasing the virulence factor from the precursor, enabling it to be secreted. The active site residues in family N4 asparagine peptide lyases are N1100, Y1227, E1249 and R1282.
Family N6 includes autoprocessing endopeptidases involved in type III protein secretion system, in which autoproteolysis is essential for mediating the secretion of proteins. Type III secretion system secretes proteins directly into host cells by an injectisome, a hollow tubular structure that penetrates into the host cell. Secreted proteins can pass through the injectisome into the host cell cytoplasm. The conserved active site residue in family N6 asparagine peptide lyases is N263.
BQUB16-Mmiranda (talk) 09:50, 22 October 2016 (UTC)
Intein-containing proteins[edit]
See also[edit]
- Asparagine
- Proteolytic enzyme
- Protease
- Lyase
- Serine proteases
- Cysteine proteases
- Threonine proteases
- Aspartic proteases
- Glutamic proteases
- Metalloproteases
- Protein precursor
- Nucleophilic substitution
- Intein
References[edit]
- ^ Rawlings ND, Barret AJ, Bateman A. Asparagine peptide lyases: a seventh catalytic type of proteolytic enzymes. (2011 Nov 4) J Biol Chem. 286(44), 38321-8 PMID 21832066
- ^ Tajima, N., Kawai, F., Park, S. Y., Tame, J. R. [[A novel intein-like autoproteolytic mechanism in autotransporter proteins. (2010) J. Mol. Biol. 402(4), 645-656 PMID 20615416
- ^ Rawlings ND, Barret AJ, Bateman A. Asparagine peptide lyases: a seventh catalytic type of proteolytic enzymes. (2011 Nov 4) J Biol Chem. 286(44), 38321-8 PMID 21832066
- ^ Rawlings ND, Barret AJ, Bateman A. Asparagine peptide lyases: a seventh catalytic type of proteolytic enzymes. (2011 Nov 4) J Biol Chem. 286(44), 38321-8 PMID 21832066
- ^ Tajima, N., Kawai, F., Park, S. Y., Tame, J. R. [[A novel intein-like autoproteolytic mechanism in autotransporter proteins. (2010) J. Mol. Biol. 402(4), 645-656 PMID 20615416
- ^ Rawlings ND, Barret AJ, Bateman A. Asparagine peptide lyases: a seventh catalytic type of proteolytic enzymes. (2011 Nov 4) J Biol Chem. 286(44), 38321-8 PMID 21832066
- ^ Rawlings, N.D., Barrett, A.J. & Finn, R.D. (2016) Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 44, D343-D350
- ^ Tajima, N., Kawai, F., Park, S. Y., Tame, J. R. [[A novel intein-like autoproteolytic mechanism in autotransporter proteins. (2010) J. Mol. Biol. 402(4), 645-656 PMID 20615416
- ^ Dautin, N., Barnard, T. J., Anderson, D. E., and Bernstein, H. D. (2007) EMBO J. 26, 1942-1952
- ^ J. March, Advanced Organic Chemistry, 4th ed., Wiley, New York, 1992
- ^ Dehart, M. P., and Anderson, B. D. (2007) J. Pharm. Sci. 96, 2667-2685
- ^ R. A. Rossi, R. H. de Rossi, Aromatic Substitution by the SRN1 Mechanism, ACS Monograph Series No. 178, American Chemical Society, 1983
- ^ Rawlings, N.D., Barrett, A.J. & Finn, R.D. (2016) Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 44, D343-D350
- ^ Wells TJ, Tree JJ, Ulett GC, Schembri MA. Autotransporter proteins: novel targets at the bacterial cell surface. (2007) 274(2), 163-72
- ^ Rawlings, N.D., Barrett, A.J. & Finn, R.D. (2016) Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 44, D343-D350
External links[edit]

