User:Bermanel
Unhydrolysable Glucose Polymers Glucans are polysaccharide of glucose monomers linked by glycosidic bonds. Accordingly, polymers built up of carbohydrate units bounded other than glycosodic bond are not considered as polysaccharides. Only four different types glucose-based polysaccharides are possible to be built: 1,6- (Starch), 1,4- (Cellulose), 1,3- (Laminarin) and 1,2-bounded glucans.


Levoglucosan is 1,6-anhydro derivative of glucose. It is one of the key products in thermal conversion of polysaccharides. Polysaccharides are not volatile at combustion temperature and propagation of fire in volume takes place due to combustion of volatile product of degradation of polysaccharides, mainly Levoglucosan. The position 2, 3, and 4 atoms carbon of Levoglucosan are available for design of polymers, which doesn’t belong to class of polysaccharides
The first representatives of main chain unhydrolysable linear polymer made up of Levoglucosan units were synthesized in 1985 by anionic polymerization of 2,3-epoxy derivatives of Levoglucosan (1,6;2,3-dianhydro-4-O-alkyl--D-mannopyranoses).

. A wide range of unique monomers with different radical R can be synthesized according to [1].There were synthesized polymers with R= -CH3 [2] -CH2CHCH2 [3], and -CH2C6H5 [4]. Investigation of polymerization kinetic of those derivatives, polymers molecular weights and molecular-weight distributions showed that the polymerization has feature of Living Polymerization System. Process takes place without termination and transfer polymer chain with degree polymerization equal mole ratio monomer to initiator [5][6]. Accordingly, the upper value molecular weight polymer limits only degree of purification system what determine the presence the system uncontrollable amount of terminators of polymer chains. Poly(2-3)-D-glucose was synthesized proceeds by transformation of Benzyl (R= -CH2C6H5) functionalized polymer.

Polymerization of 3,4-epoxy Levoglucosan (1,6;3,4-dianhydro-2-O-alkyl--D-galactopyranose) [7] results in formation 3,4-bounded Levoglucosan polymer
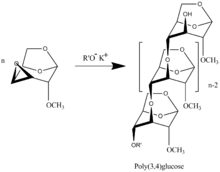
The presence of 1,6-anhydro structure in every unit of polymer chains allows to apply all specter of well developed methods of carbohydrate chemistry with formation of highly intriguing biological application polymers. A review on synthesis and physical properties of synthesized polymers is presented in [8]. The polymers are the only known regular polyethers built up of carbohydrate units in main polymer chain [9]. 'Bold text--Bermanel (talk) 17:11, 2 May 2011 (UTC)--Bermanel (talk) 17:11, 2 May 2011 (UTC)
- ^ Carlson L. J., “Preparation of 2- and 4-Substituted D-Glucose Derivatives from 1,6-Anhydro-β-D- glucopyranose” J. Org. Chem., 1965, 30 (11), pp 3953–3955
- ^ Berman, E.L., Gorkovenko A.A., Zubov, V.P., and Ponomarenko, V.A.,"Regio and Stereospecific Synthesis of Polyglucose with Novel Type BondSoviet J.Bioorg. Chem. 11 (1985), 1125-1129
- ^ Gorkovenko, A.A., Berman, E.L., and Ponomarenko, V.A. Polymerization of 1, 6;2,3 dianhydro 4 O allyl D manno¬pyranose" Vysocomol. Soed., Ser. B, 1987, 29, 134 137
- ^ Gorkovenko, A.A., Berman, E.L., and Ponomarenko, V.A. "A New Polymer of Glucose. Poly(2 3) D glucose" Soviet J. Bioorg. Chem., 1987, 13, 218 222
- ^ Berman, E.L., Gorkovenko, A.A., Rogozhkina, E.D., Izumnikov, A.A., and Ponomarenko, V.A. “Kinetics and Mechanism of Epoxy Ring-Opening Polymerization of 1,6;2,3-Dianhydro-4-O-alkyl--D-mannopyranoses” Polymer Sci. USSR, 1988, 413-418
- ^ Berman E.L. Gorkovenko, A.A., Rogozhkina, E.D., Izyumnikov, A.L., and Ponomarenko, V.A. "Synthesis of Chiral Derivatives of Poly(Ethylene Oxide)" Bull. Acad. Sci. USSR, Div. Chem. Sci., 1988, 705 707
- ^ Gorkovenko A. A., Berman, E.L., and Ponomarenko, V.A., Poly(3 4) 2 O methyl 1,6 anhydro D glucopyranose. The First Example of (3 4) linked Polymer Carbohydrates" Soviet J. Bioorg. Chem. 12 (1986), 514-520
- ^ Berman, E.L., “New Glucose Polymers” in “Levoglucosenone and Levoglucosanes: Symposium: 204th National meeting”, Zbigniew J. Witczak (editor), American Chemical Society. Division of Carbohydrate Chemistry, 189-214. Publisher: A T L Press, Scientific Publishers ISBN-13: 9781882360130 ISBN: 1882360133
- ^ Berman E.L., Gorkovenko, A.A., and Ponomarenko, V.A. "Structure and Polymerizability of 1,6;2,3 and 1,6;3,4¬ Dianhydrohexapyranoses" Polymer Sci. USSR, 1988, 30, 497¬-502
