User:Chem8240ds/sandbox

In chemistry, aurophilicity refers to the tendency of gold complexes to aggregate via formation of weak gold-gold bonds.[2][1]
Overview[edit]
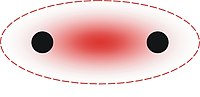
The phenomenon of aurophilicity is most commonly observed crystallographically for Au(I) compounds. The aurophilic bond has a length of about 3.0 Å and a strength of about 7-12 kcal/mol[1], which is comparable to the strength of a hydrogen bond. The aurophilic interaction is thought to result from electron correlation of the closed-shell components, which is unusual in light of the fact that closed-shell atoms generally have negligible interaction with one another at distances on the scale of the Au-Au bond. This is somewhat similar to van der Waals interactions, but is unusually strong due to relativistic effects. Observations and theory show that, on average, 28% of the binding energy in aurophilic interaction can be attributed to relativistic expansion of the gold d orbitals.[3]
Another important feature of aurophilicity is the propensity of gold atoms to aggregate around nucleation sites--specifically, though not limited to, ligands that bind through phosphorus, nitrogen, and sulfur centers. While both intra- and inter-molecular aurophilic interactions exist, only intramolecular aggregation has been observed at such nucleation sites.[4]
Applications[edit]
The similarity in strength between hydrogen bonding and aurophilic interaction has proven to be a convenient tool in the field of polymer chemistry. There has been much research into self-assembling supermolecular structures, both those that aggregate by aurophilicity alone and those that contain both aurophilic and hydrogen-bonding interactions.[5] An important and exploitable property of aurophilic interactions relevant to their supermolecular chemistry is that while both inter- and intramolecular interactions are possible, intermolecular aurophilic linkages are comparatively weak and easily broken by solvation; most complexes that exhibit intramolecular aurophilic interactions retain such moieties in solution.[1]

Other Phenomena[edit]
Similar metallophilic interactions exist for a few other heavy metals, such as mercury, and can also be observed between atoms of different elements. Some documented examples include Hg(II)-Au(I), Hg(II)-Pt(II), and Hg(II)-Pd(II).[6] In accordance with theoretical calculations, which predict a local maximum for relevant relativistic effects for gold atoms, none of these other interactions are as strong as aurophilicity.[1][7]
References[edit]
- ^ a b c d e f Hubert Schmidbaur (2000). "The Aurophilicity Phenomenon: A Decade of Experimental Findings, Theoretical Concepts and Emerging Application". Gold Bulletin. 33 (1): 3–10.
- ^ Hubert Schmidbaur (1995). "Ludwig Mond Lecture. High-carat gold compounds". Chem. Soc. Rev. 24: 391–400. doi:10.1039/CS9952400391.
- ^ Nino Runeberg, Martin Schütz, and Hans-Joachim Werner (1999). "The aurophilic attraction as interpreted by local correlation methods". J. Chem. Phys. 110: 7210–7215. doi:10.1063/1.478665.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hubert Schmidbaur, Stephanie Cronje, Bratislav Djordjevic, and Oliver Schuster (2005). "Understanding gold chemistry through relativity". J. Chem. Phys. 311: 151–161. doi:10.1016.
{{cite journal}}: Check|doi=value (help)CS1 maint: multiple names: authors list (link) - ^ William J. Hunks, Michael C. Jennings, and Richard J. Puddephatt (2002). "Supramolecular Gold(I) Thiobarbiturate Chemistry: Combining Aurophilicity and Hydrogen Bonding to Make Polymers, Sheets, and Networks". Inorg. Chem. 41: 4590–4598. doi:10.1021/ic020178h.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mieock Kim, Thomas J. Taylor, and François P. Gabbai. Hg(II)···Pd(II) Metallophilic Interactions. J. Am. Chem. Soc. 2008, 130, 6332–6333. doi:10.1021/ja801626c
- ^ Behnam Assadollahzadeh and Peter Schwerdtfege (2008). "A comparison of metallophilic interactions in group 11[X–M–PH3]n (n = 2–3) complex halides (M = Cu, Ag, Au; X = Cl, Br, I) from density functional theory". Chemical Physics Letters. 462: 222–228. doi:10.1016/j.cplett.2008.07.096.