User:Cmrufo/sandbox
Metal complexes are important intermediates in organometallic catalytic reactions. A few examples of such catalytic cycles are allylic substitution, allylic oxidation and 1,4- oxidation of conjugated dienes. Metal complexes must have ligands (some of which can be pi ligands) attached that can undergo reactions.
Addition to Pi Ligands (Davies, Green, Mingos Rules)[edit]
Davies, Green, and Mingos have studied additions to cationic metal complexes. They have compiled rules that summarize where a nucleophile will add on the pi ligand.
Nucleophilic attack occurs on even before odd coordinated polyenes. Nucleophilic attack occurs on open before closed polyenes. For even, open systems addition occurs at the terminal position. For odd, open addition occurs at the terminal position only if an electron withdrawing metal fragment is present.
Simplified: even before odd and open before closed
Examples of Pi Ligand systems [1][edit]
The even odd classification is based on hapticity and electron count assuming that the ligand is neutral. The following are examples of these pi ligands.
Certain pi ligands are more reactive than others. The following shows which pi ligands are more reactive and which are less reactive.

Examples of complexes[edit]
The following are examples of complexes that obey the rules.

Rule 1:[edit]
For reactions under charge control even ligands have a HOMO that is doubly occupied. These two electrons transfer from the ligand to the metal resulting in a ligand charge of 2+. This makes the ligand susceptible to attack.
For odd ligands the HOMO is singly occupied therefore only one electron is transferred resulting in a 1+ charge on the ligand.
A 2+ charge is going to be more susceptible to attack than a 1+ charge. Even ligands have this 2+ charge.
Rule 2:[edit]
For closed pi systems the positive charge on the ring is more disturbed on the aromatic ring because of symmetry. This means that closed systems have less positive charge, which means they are less susceptible to attack.
Rule 3: Regiochemistry of attack[edit]
Attack occurs at the terminal position when the ligand is even and open. [2]
The metal center is electron withdrawing and has positive nature. This would produce the effect is if the ligand were a carbonyl. Electron poor metals cannot back bond well to the carbonyl. The more electron withdrawing the metal is the more triple bond nature the CO has. This means that there is a higher force constant. The force constant for the CO represents the the same force constant for pi ligands if they replace COs. Nucleophilic addition does not happen if kCO* (effective force constant) is below the threshold value [3]
The following figure shows a metal attached to a carbonyl group, this group has a δ+ charge and therefore is more suspectible to attack. If this was replaced with the pi ligand it would also be suspectiable to attack.
Example of Rule 3 [4][edit]
Incoming nucleophilic attack happens on the largest lobe of the LUMO.

In this example the ring system can be thought of as analogous to butadiene. The LUMO of butadiene has larger lobes in the middle rather than the terminal positions. This means attack will occur at the internal positions.
Internal attack [5] [6][edit]
Here the ligand that is already attached to the metal acts as the nucleophile and attacks the metal center internally.

Effects of types of ligands on regiochemistry of attack[edit]
Nucleophilic attack at terminal position of allyl ligands when pi accepting ligand is present [7].
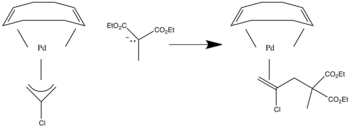
If sigma donating ligands are present they pump electrons into the ligand and attack occurs at the internal position.

Effects of asymmetrical ligands[edit]
When asymmetrical allyl ligands are present attack occurs at the more substituted position [8].

In this case the attack will occur on the carbon with both R groups attached to it since that is the more substituted position.
Effects of large pi ligands[edit]
When large pi ligands [9] are present they can undergo different kinds of nucleophilic attacks. In the following figure the nucleophilic attack can occur from either the top or bottom and reduce the double bond and add the nucleophile.

This nucleophilic attack can occur at either the top or bottom also and add the nucelophile.

Uses in synthesis[edit]
Nucleophilic addition to pi ligands can be used in synthesis. One example of this is to make cyclic metal compounds [10]. Nucleophiles add to the center of the pi ligand and produces a metallobutane.


References[edit]
- ^ [Spessard, Gary O., and Gary L. Miessler. Organometallic Chemistry. 2nd ed. New York: Oxford, 2010 PP 270-285 ISBN 978-0-19-533099-1]
- ^ S.G. Davies, M. L. H. Green, and D. M. P. Mingos, Tetrahedron 1978, 34, 3047
- ^ R. C. Bush and R. J. Angelici, J. Am. Chem. Soc. 1986, 108, 2133
- ^ J.-S. Fan and R.-S. Liu, Organometallics 1998, 17, 1002
- ^ R. A. Periana and R. G. Bergman, J. Am. Chem. Soc. 1984, 106, 7272
- ^ T. Suzuki, G. Okada, Y. Hioki, and H. Fujimoto, Organometallics 2003, 22, 3649
- ^ A. Aranyos, K. J. Szabo, A. M. Castano, and J.-E. Backvall. Organometallics 1997, 16, 1056
- ^ F. Delbecq and C. Lapouge, Organometallics 2000, 19, 2716
- ^ S. Scho ̈rshusen and J. Heck, Organometallics 2007, 26, 5386
- ^ R. Periana and R. Bergman, J. Am. Chem. Soc. 1986, 108, 7346


