User:DJIndica/Sandbox3
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number.[1] These atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields,[2] long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei.[3] The core electrons shield the outer electron from the electric field of the nucleus such that, from a distance, the electric potential looks identical to that experienced by the electron in a hydrogen atom.[4]
In spite of its shortcomings, the Bohr model of the atom is useful in explaining these properties. Classically an electron in a circular orbit of radius r, about a nucleus of charge +e, obeys Newton's second law:
where k = 1/(4πε0).
Orbital momentum is quantized in units of ħ:
- .
Combining these two equations leads to Bohr's expression for the orbital radius in terms of the principal quantum number, n:
It is now apparent why Rydberg atoms have such peculiar properties; the radius of the orbit scales as n2 (the n = 137 state of hydrogen has an atomic radius ~1 µm) and the geometric cross-section as n4. Thus Rydberg atoms are extremely large with loosely bound valence electrons, easily perturbed or ionized by collisions or external fields.
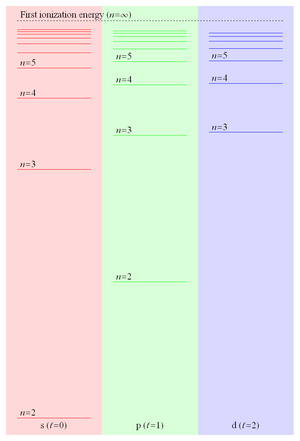
Because the binding energy of a Rydberg electron is proportional to 1/r and hence falls off like 1/n2, the energy level spacing falls off like 1/n3 leading to ever more closely spaced levels converging on the first ionization energy. These closely spaced Rydberg states form what is commonly referred to as the Rydberg series. Figure 1 shows some of the energy levels of the lowest three values of orbital angular momentum in lithium.
History[edit]
The existence of the Rydberg series was first demonstrated in 1885 when Johann Balmer discovered a simple empirical formula for the wavelengths of light associated with transitions in atomic hydrogen. Three years later the Swedish physicist Johannes Rydberg presented a generalized and more intuitive version of Balmer's formula that came to be known as the Rydberg formula. This formula indicated the existence of an infinite series of ever more closely spaced discrete energy levels converging on a finite limit.[5]
This series was qualitatively explained in 1913 by Niels Bohr with his semiclassical model of the hydrogen atom in which quantized values of angular momentum lead to the observed discrete energy levels. A full quantitative derivation of the observed spectrum was derived by Wolfgang Pauli in 1926 following development of quantum mechanics by Werner Heisenberg and others.
Production of Rydberg atoms[edit]
The only truly stable state of an atom is the ground state with n = 1. The study of Rydberg states requires a reliable technique for exciting ground state atoms to states with a large value of n.
Electron impact excitation[edit]
Much early experimental work on Rydberg atoms relied on the use of collimated beams of fast electrons incident on ground-state atoms.[6] Inelastic scattering processes can use the electron kinetic energy to increase the atoms' internal energy exciting to a broad range of different states including many high-lying Rydberg states,
- .
Because the electron can retain any arbitrary amount of its initial kinetic energy this process always results in a population with a broad spread of different energies.
Charge exchange excitation[edit]
Another mainstay of early Rydberg atom experiments relied in charge exchange between a beam of ions and a population of neutral atoms of another species resulting in the formation of a beam of highly excited atoms,[7]
- .
Again, because the kinetic energy of the interaction can contribute to the final internal energies of the constituents this technique populates a broad range of energy levels.
Optical excitation[edit]
The arrival of tunable dye lasers in the 1970s allowed a much greater level of control over populations of excited atoms. In optical excitation the incident photon is absorbed by the target atom, absolutely specifying the final state energy. The problem of producing single state, mono-energetic populations of Rydberg atoms thus becomes the somewhat simpler problem of precisely controlling the frequency of the laser output,
- .
This form of direct optical excitation is generally limited to experiments with the alkali metals because the ground state binding energy in other species is too high to be accessible with most laser systems.
For atoms with a large valence electron binding energy (equivalent to a large first ionization energy) the excited states of the Rydberg series are inaccessible with conventional laser systems. Initial collisional excitation can make up the energy shortfall allowing optical excitation to be used to select the final state. Although the initial step excites to a broad range of intermediate states, the precision inherent in the optical excitation process means that the laser light only interacts with a specific subset of atoms in a particular state, exciting to the chosen final state.
Hydrogenic potential[edit]

An atom in a Rydberg state has a valence electron in a large orbit far from the ion core; in such an orbit the outermost electron feels an almost hydrogenic, Coulomb potential, UC from a compact ion core consisting of a nucleus with Z protons and the lower electron shells filled with Z-1 electrons. An electron in the spherically symmetric Coulomb potential has potential energy:
- .
The similarity of the effective potential ‘seen’ by the outer electron to the hydrogen potential is a defining characteristic of Rydberg states and explains why the electron wavefunctions approximate to classical orbits in the limit of the correspondence principle.[3] There are three notable exceptions that can be characterized by the additional term added to the potential energy:
- An atom may have two (or more) electrons in highly excited states with comparable orbital radii. In this case the electron-electron interaction gives rise to a significant deviation from the hydrogen potential.[8] For an atom in a multiple Rydberg state, the additional term, Uee, includes a summation of each pair of highly excited electrons:
- .
- If the valence electron has very low angular momentum (interpreted classically as an extremely eccentric elliptical orbit) then it may pass close enough to polarise the ion core, giving rise to a 1/r4 core polarization term in the potential.[9] The interaction between an induced dipole and the charge that produces it is always attractive so this contribution is always negative,
- ,
- where αd is the dipole polarizability. Figure 2 shows how the polarization term modifies the potential close to the nucleus.
- If the outer electron penetrates the inner electron shells, it will 'see' more of the charge of the nucleus and hence experience a greater force. In general the modification to the potential energy is not simple to calculate and must be based on knowledge of the geometry of the ion core.[10]
Quantum mechanics of Rydberg atoms[edit]
Quantum mechanically a state with high n refers to an atom in which the valence electron(s) have been excited into a formerly unpopulated electron orbital with higher energy and lower binding energy. In hydrogen the binding energy is given by:
- ,
where Ry = 13.6 eV is the Rydberg constant. The low binding energy at high values of n explains why Rydberg states are susceptible to ionization.
Additional terms in the potential energy expression for a Rydberg state, on top of the hydrogenic Coulomb potential energy require the introduction of a quantum defect,[4] δl, into the expression for the binding energy:
- .
The quantum defect is strongly dependent on the orbital angular momentum with low l states spending more time in the vicinity of the ion-core leading to significant deviation
Rydberg state lifetimes[edit]
Rydberg electron wavefunctions[edit]
The long lifetimes of Rydberg states with high orbital angular momentum can be explained in terms of the overlapping of wavefunctions. The wavefunction of an electron in a high l state (high angular momentum, 'circular orbit') has very little overlap with the wavefunctions of the inner electrons and hence remains relatively unperturbed.
The three exceptions to the definition of a Rydberg atom as an atom with a hydrogenic potential, have an alternative, quantum mechanical description that can be characterized by the additional term(s) in the atomic Hamiltonian:
- If a second electron is excited into a state ni, energetically close to the state of the outer electron no, then its wavefunction become almost as large as the first (a double Rydberg state). This occurs as ni approaches no and leads to a condition where the size of the two electron’s orbits are related;[8] a condition sometimes referred to as radial correlation.[1] An electron-electron repulsion term must be included in the atomic Hamiltonian.
- Polarization of the ion core produces an anisotropic potential that causes an angular correlation between the motions of the two outermost electrons.[1][11] This can be thought of as a tidal locking effect due to a non-spherically symmetric potential. A core polarization term must be included in the atomic Hamiltonian.
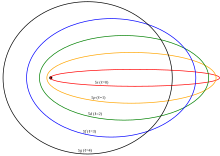
- The wavefunction of the outer electron in states with low orbital angular momentum l, is periodically localised within the shells of inner electrons and interacts with the full charge of the nucleus.[10] Figure 3 shows a semi-classical interpretation of angular momentum states in an electron orbital, illustrating that low-l states pass closer to the nucleus potentially penetrating the ion core. A core penetration term must be added to the atomic Hamiltonian.
Rydberg atoms in external fields[edit]
The large separation between the electron and ion-core in a Rydberg atom makes possible an extremely large electric dipole moment, d. There is an energy associated with the presence of an electric dipole in an electric field, F, known in atomic physics as a Stark shift,
Depending on the sign of the projection of the dipole moment onto the local electric field vector a state may have energy that increases or decreases with field strength (low-field and high-field seeking states respectively). The narrow spacing between adjacent n-levels in the Rydberg series means that states can approach degeneracy even for relatively modest field strengths. The theoretical field strength at which a crossing would occur assuming no coupling between the states is given by the Inglis-Teller limit,[13]
In the hydrogen atom, the pure 1/r Coulomb potential does not couple Stark states from adjacent n-manifolds resulting in real crossings as shown in figure 4. The presence of additional terms in the potential energy can lead to coupling resulting in avoided crossings as shown for lithium in figure 5.
What makes Rydberg atoms worth studying?[edit]
Investigating diamagnetic effects[edit]
The large sizes and low binding energies of Rydberg atoms lead to a high magnetic susceptibility, Χ. As diamagnetic effects scale with the area of the orbit and the area is proportional to the radius squared (A α n4), effects impossible to detect in ground state atoms become obvious in Rydberg atoms, which demonstrate very large diamagnetic shifts.[14]
Rydberg atoms in plasmas[edit]
Rydberg atoms form commonly in plasmas due to the recombination of electrons and positive ions; low energy recombination results in fairly stable Rydberg atoms, while recombination of electrons and positive ions with high kinetic energy often form autoionising Rydberg states. Rydberg atoms’ large sizes and susceptibility to perturbation and ionisation by electric and magnetic fields, are an important factor determining the properties of plasmas.[15]
Condensation of Rydberg atoms forms Rydberg matter most often observed in form of long-lived clusters. The de-excitation is significantly impeded in Rydberg matter by exchange-correlation effects in the non-uniform electron liquid formed on condensation by the collective valence electrons, which causes extended lifetime of clusters.[16]
Rydberg atoms in astrophysics[edit]
In the time between the early absorption spectroscopy experiments and the arrival of tunable lasers, interest in Rydberg atoms was kept alive by the realisation that they are common in interstellar space, and as such are an important radiation source for astronomers.[17]
The density within interstellar gas clouds is typically many orders of magnitude lower than the best laboratory vacuums attainable on Earth, allowing Rydberg atoms to persist for long periods of time without being ionised by collisions or electric and magnetic fields. As a result of this longevity and the abundance of hydrogen it is particularly common for astronomers to observe radiation from the heavens at a frequency of 2.4 GHz, now known to correspond to the hydrogen n = 109 to n = 108 transition.[18] Such a highly excited hydrogen atom on Earth would be ionised almost immediately as the binding energy would be significantly below thermal energies.
Strongly interacting systems[edit]
Due to their large size, Rydberg atoms can exhibit very large electric dipole moments. Calculations using perturbation theory show that this results in strong interactions between two close Rydberg atoms. Coherent control of these interactions combined with the their relatively long lifetime makes them a suitable candidate to realize a quantum computer.[19] As of March 2009[update], a two-qubit gate has not been achieved experimentally; however, observations of collective excitations or conditional dynamics have been reported, both between two individual atoms [20] [21] and in mesoscopic samples.[22] Strongly interacting Rydberg atoms also feature quantum critical behavior, which makes them interesting to study on their own.[23]
Classical simulation of a Rydberg atom[edit]


A simple 1/r potential results in a closed Keplerian elliptical orbit. In the presence of an external electric field Rydberg atoms can obtain very large electric dipole moments making them extremely susceptible to perturbation by the field. Figure 6 shows how application of an external electric field (known in atomic physics as a Stark field) changes the geometry of the potential, dramatically changing the behaviour of the electron. A Coulombic potential does not apply any torque as the force is always antiparallel to the position vector (always pointing along a line running between the electron and the nucleus):
- ,
- .
With the application of a static electric field, the electron feels a continuously changing torque. The resulting trajectory becomes progressively more distorted over time, eventually going through the full range of angular momentum from L = LMAX, to a straight line L=0, to the initial orbit in the opposite sense L = -LMAX.[24]
The time period of the oscillation in angular momentum (the time to complete the trajectory in figure 7), almost exactly matches the quantum mechanically predicted period for the wavefunction to return to its initial state, demonstrating the classical nature of the Rydberg atom.
See also[edit]
References[edit]
- ^ a b c Gallagher, Thomas F. (1994). Rydberg Atoms. Cambridge: Cambridge University Press. ISBN 0521021669.
- ^ Metcalf Research Group (2004-11-08). "Rydberg Atom Optics". Stoney Brook University. Retrieved 2008-07-30.
- ^ a b J. Murray-Krezan (2008). "The classical dynamics of Rydberg Stark atoms in momentum space". American Journal of Physics. 76 (11): 1007–1011. doi:10.1119/1.2961081. Cite error: The named reference "Classical" was defined multiple times with different content (see the help page).
- ^ a b Nolan, James (2005-05-31). "Rydberg Atoms and the Quantum Defect". Davidson College. Retrieved 2008-07-30.
- ^ I. Martinson and L. J. Curtis (2005). "Janne Rydberg – his life and work". Nuclear Instruments and Methods in Physics Research Section B. 235 (1–4): 17–22. doi:10.1016/j.nimb.2005.03.137.
- ^ J. Olmsted (1967). "Excitation of nitrogen triplet states by electron impact". Radiation Research. 31 (2): 191–200. doi:10.2307/3572319. JSTOR 3572319. PMID 6025857.
- ^ M. Haugh; et al. (1966). "Electronic excitation accompanying charge exchange". Journal of Chemical Physics. 44 (2): 837–839. doi:10.1063/1.1726773.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b I. K. Dmitrieva and G. I. Plindov (1993). "Energies of Doubly Excited Sates. The Double Rydberg Formula". Journal of Applied Spectroscopy. 59 (1–2): 466–470. doi:10.1007/BF00663353. S2CID 96628309.
- ^ L. Neale and M. Wilson (1995). "Core Polarization in Kr VIII". Physical Review A. 51 (5): 4272–4275. doi:10.1103/PhysRevA.51.4272. PMID 9912104.
- ^ a b C. E. Theodosiou (1983). "Evaluation of penetration effects in high-l Rydberg states". Physical Review A. 28 (5): 3098–3101. doi:10.1103/PhysRevA.28.3098.
- ^ T. A. Heim and A. R. P. Rau (1995). "Excitation of high-lying pair-Rydberg states". Journal of Physics B: Atomic, Molecular and Optical Physics. 28 (24): 5309–5315. doi:10.1088/0953-4075/28/24/015.
- ^ a b M. Courtney; et al. (1995). "Classical, semiclassical, and quantum dynamics of lithium in an electric field". Physical Review A. 51 (5): 3604–3620. doi:10.1103/PhysRevA.51.3604. PMID 9912027.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ D.R. Inglis and E. Teller (1939). "Ionic Depression of Series Limits in One-Electron Spectra". Astrophysical Journal. 90: 439. doi:10.1086/144118.
- ^ J. Neukammer; et al. (1984). "Diamagnetic shift and singlet-triplet mixing of 6snp Yb Rydberg states with large radial extent". Physical Review A. 30 (2): 1142–1144. doi:10.1103/PhysRevA.30.1142.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ G. Vitrant; et al. (1982). "Rydberg to plasma evolution in a dense gas of very excited atoms" (PDF). Journal of Physics B: Atomic and Molecular Physics. 15 (2): L49–L55. doi:10.1088/0022-3700/15/2/004.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ E. A. Manykin; et al. (2006). "Rydberg matter: properties and decay". Proceedings of the SPIE. 6181: 618105:1–9. doi:10.1117/12.675004. S2CID 96732651.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Y. N. Gnedin; et al. (2009). "Rydberg atoms in astrophysics". New Astronomy Reviews. 53 (7–10): 259–265. arXiv:1208.2516. doi:10.1016/j.newar.2009.07.003. S2CID 119276100.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ J. Poutanen (2007-03-22). "Gasdynamics and Interstellar Medium" (PDF). University of Oulu. Retrieved 2008-07-31.
- ^ D. Jaksch; et al. (2000). "Fast Quantum Gates for Neutral Atoms". Physical Review Letters. 85 (10): 2208–2211. arXiv:quant-ph/0004038. doi:10.1103/PhysRevLett.85.2208. PMID 10970499. S2CID 16713798.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ A. Gaëtan; et al. (2009). "Observation of collective excitation of two individual atoms in the Rydberg blockade regime". Nature Physics. 5 (2): 115–118. arXiv:0810.2960. doi:10.1038/nphys1183. S2CID 17645519.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ E. Urban; et al. (2009). "Observation of Rydberg blockade between two atoms". Nature Physics. 5 (2): 110–114. arXiv:0805.0758. doi:10.1038/nphys1178. S2CID 6220176.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ R. Heidemann; et al. (2007). "Evidence for Coherent Collective Rydberg Excitation in the Strong Blockade Regime". Physical Review Letters. 99 (16): 163601. arXiv:quant-ph/0701120. doi:10.1103/PhysRevLett.99.163601. PMID 17995249. S2CID 13622806.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ H. Weimer; et al. (2008). "Quantum Critical Behavior in Strongly Interacting Rydberg Gases". Physical Review Letters. 101 (25): 250601. arXiv:0806.3754. doi:10.1103/PhysRevLett.101.250601. PMID 19113686. S2CID 28636728.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ T. P. Hezel; et al. (1992). "Classical view of the Stark effect in hydrogen atoms". American Journal of Physics. 60 (4): 324–328. doi:10.1119/1.16875.
{{cite journal}}: Explicit use of et al. in:|author=(help)

















