User:Dwong527/sandbox
| Phosphoglycerate kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC no. | 2.7.2.3 | ||||||||
| CAS no. | 9001-83-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Phosphoglycerate kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of yeast phosphoglycerate kinase.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | PGK | ||||||||
| Pfam | PF00162 | ||||||||
| InterPro | IPR001576 | ||||||||
| PROSITE | PDOC00102 | ||||||||
| SCOP2 | 3pgk / SCOPe / SUPFAM | ||||||||
| |||||||||
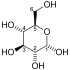
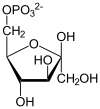
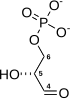
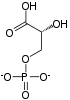
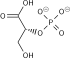
Phosphoglycerate kinase (EC 2.7.2.3) (PGK) is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP. Like all kinases it is a transferase. PGK is a major enzyme used in glycolysis, in the first ATP-generating step of the glycolytic pathway. In gluconeogenesis, the reaction catalyzed by PGK proceeds in the opposite direction, generating ADP and 1,3-BPG. Not all variants of glycolysis use PGK.
In humans, two isozymes of PGK have been so far identified, PGK1 and PGK2. The isoenzymes have 87-88% identical amino acid sequence identity and though they are structurally and functionally similar, they have different localizations: PGK2, encoded by an autosomal gene, is unique to meiotic and postmeiotic spermatogenic cells, while PGK1, encoded on the X-chromosome, is ubiquitously expressed in all cells.[2]
Biological function[edit]

PGK is present in all living organisms as one of the two ATP-generating enzymes in glycolysis. In the gluconeogenic pathway, PGK catalyzes the reverse reaction. Under biochemical standard conditions, the glycolytic direction is favored.[1]
In the Calvin cycle in photosynthetic organisms, PGK catalyzes the phosphorylation of 3-PG, producing 1,3-BPG and ADP, as part of the reactions that regenerate ribulose-1,5-bisphosphate.
PGK has been reported to exhibit thiol reductase activity on plasmin, leading to angiostatin formation, which inhibits angiogenesis and tumor growth. The enzyme was also shown to participate in the DNA replication and repair in mammal cell nuclei.[3]
The human isozyme PGK2, which is only expressed during spermatogenesis, was shown to be essential for sperm function in mice.[4]
Structure[edit]
Overview[edit]
PGK is found in all living organisms and its sequence has been highly conserved throughout evolution. The enzyme exists as a 415-residue monomer containing two nearly equal-sized domains that correspond to the N- and C-termini of the protein.[5] 3-phosphoglycerate (3-PG) binds to the N-terminal, while the nucleotide substrates, MgATP or MgADP, bind to the C-terminal domain of the enzyme. This extended two-domain structure is associated with large-scale 'hinge-bending' conformational changes, similar to those found in hexokinase.[6] The two proteins of the domain are separated by a cleft and linked by two alpha-helices.[2] At the core of each domain is a 6-stranded parallel beta-sheet surrounded by alpha helices. The two lobes are capable of folding independently, consistent with the presence of intermediates on the folding pathway with a single domain folded.[7][8] Though the binding of either substrate triggers a conformational change, only the concerted binding of both substrates allows domain closure then transfer of the phosphoryl group.[2]
The enzyme has a tendency to exist in the open conformation with short periods of closure and catalysis, which allow for rapid diffusion of substrate and products through the binding sites; the open conformation of PGK is more conformationally stable due to the exposure of a hydrophobic region of the protein upon domain closure.[7]
Role of magnesium[edit]
Magnesium ions are normally complexed to the phosphate groups the nucleotide substrates of PGK. It is known that in the absence of magnesium, no enzyme activity occurs. [9] The The bivalent metal assists the enzyme ligands in shielding the bound phosphate group's negative charges, allowing the nucleophilic attack to occur; this charge-stabilization is a typical characteristic of phospho-transfer reaction.[10] It is theorized that the ion may also encourage domain closure when PGK has bound both substrates.[9]
Mechanism[edit]

Without either substrate bound, PGK exists in an "open" conformation. After both the triose and nucleotide substrates are bound to the N- and C-terminal domains, respectively, an extensive hinge-bending motion occurs, bringing the domains and their bound substrates into close proximity and leading to a "closed" conformation.[11] Then, in the case of the forward glycolytic reaction, the beta-phosphate of ADP initiates a nucleophilic attack on the 1-phosphate of 1,3-BPG in an SN2 substitution reaction.[12] The Lys219 on the enzyme guides the phosphate group to the substrate.
In the glycolytic pathyway, 1,3-BPG is the phosphate donor and has a high phosphoryl-transfer potential. The PGK-catalyzed transfer of the phosphoryl group from 1,3-BPG to ADP to yield ATP is powered by the energy from the carbon-oxidation reaction of the previous glycolytic step (converting glyceraldehyde 3-phosphate to 3-phosphoglycerate).
Regulation[edit]
The enzyme is activated by low concentrations of various multivalent anions, such as pyrophosphate, sulfate, phosphate, and citrate. High concentrations of MgATP and 3-PG activates PGK, while Mg2+ at high concentrations non-competitively inhibits the enzyme. [13] PGK activity is inhibited by salicylates, which appear to mimic the enzyme's nucleotide substrate.[14]
Macromolecular crowding has been shown to increase PGK activity in both computer simluations and in vitro environments simulating a cell interior; as a result of crowding, the enzyme becomes more enyzmatically active and more compact.[5]
Disease relevance[edit]
Phosphoglycerate kinase (PGK) deficiency is an X-linked recessive trait associated with hemolytic anemia, mental disorders and myopathy in humans.[15][16] Since the trait is X-linked, it is usually fully expressed in males, who have one X chromosome; affected females are typically asymptomatic. [16][2] The condition results from mutations in Pgk1, the gene encoding PGK, and twenty mutations have been identified.[16][2] On a molecular level, the mutation in Pgk1 impairs the thermal stability and inhibits the catalytic activity of the enzyme.[2] PGK is the only enzyme in the immediate glycolytic pathway encoded by an X-linked gene. In the case of hemolytic anemia, PGK deficiency occurs in the erythrocytes. Currently, no definitive treatment exists for PGK deficiency.[17]
PGK1 overexpression has been associated with gastric cancer and has been found to increase the invasiveness of gastric cancer cells in vitro.[18] The enzyme is secreted by tumor cells and participates in the angiogenic process, leading to the release of angiostatin and the inhibition of tumor blood vessel growth.[3][19]
Human isozymes[edit]
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References[edit]
- ^ a b Watson HC, Walker NP, Shaw PJ; et al. (1982). "Sequence and structure of yeast phosphoglycerate kinase". EMBO J. 1 (12): 1635–40. doi:10.1002/j.1460-2075.1982.tb01366.x. PMC 553262. PMID 6765200.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f Chiarelli LR, Morera SM, Bianchi P, Fermo E, Zanella A, Galizzi A, Valentini G (2012). "Molecular insights on pathogenic effects of mutations causing phosphoglycerate kinase deficiency". PLOS ONE. 7 (2): e32065. doi:10.1371/journal.pone.0032065. PMC 3279470. PMID 22348148.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Lay, Angelina J.; Jiang, Xing-Mai; Kisker, Oliver; Flynn, Evelyn; Underwood, Anne; Condron, Rosemary; Hogg, Philip J. (14 December 2000). "Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase". Nature. 408 (6814): 869–873. doi:10.1038/35048596. PMID 11130727.
- ^ Danshina, Polina (1). "Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice". Biology of Reproduction. 82 (1): 136–145. doi:10.1095/biolreprod.109.079699. PMC 2802118. PMID 19759366.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Dhar, Apratim; Samiotakis, Antonios; Ebbinghaus, Simon; Nienhaus, Lea; Homouz, Dirar; Gruebele, Martin; Cheung, Margaret S. (4 October 2010). "Structure, function, and folding of phosphoglycerate kinase are strongly perturbed by macromolecular crowding". Proceedings of the National Academy of Sciences. 107 (41): 17586–17591. doi:10.1073/pnas.1006760107. PMC 2955104. PMID 20921368.
- ^ Kumar S, Ma B, Tsai CJ, Wolfson H, Nussinov R (1999). "Folding funnels and conformational transitions via hinge-bending motions". Cell Biochem. Biophys. 31 (2): 141–64. doi:10.1007/BF02738169. PMID 10593256.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Yon JM, Desmadril M, Betton JM, Minard P, Ballery N, Missiakas D, Gaillard-Miran S, Perahia D, Mouawad L (1990). "Flexibility and folding of phosphoglycerate kinase". Biochimie. 72 (6–7): 417–29. doi:10.1016/0300-9084(90)90066-P. PMID 2124145.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zerrad L, Merli A, Schröder GF, Varga A, Gráczer É, Pernot P, Round A, Vas M, Bowler MW (April 2011). "A spring-loaded release mechanism regulates domain movement and catalysis in phosphoglycerate kinase". J. Biol. Chem. 286 (16): 14040–8. doi:10.1074/jbc.M110.206813. PMC 3077604. PMID 21349853.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b Varga, Andrea; Palmai, Zoltan; Gugolya, Zoltán; Gráczer, Éva; Vonderviszt, Ferenc; Závodszky, Péter; Balog, Erika; Vas, Mária (21 December 2012). "Importance of Aspartate Residues in Balancing the Flexibility and Fine-Tuning the Catalysis of Human 3-Phosphoglycerate Kinase". Biochemistry. 51 (51): 10197–10207. doi:10.1021/bi301194t. PMID 23231058.
- ^ Cliff, Matthew J.; Bowler, Matthew W.; Varga, Andrea; Marston, James P.; Szabó, Judit; Hounslow, Andrea M.; Baxter, Nicola J.; Blackburn, G. Michael; Vas, Mária; Waltho, Jonathan P. (12). "Transition state analogue structures of human phosphoglycerate kinase establish the importance of charge balance in catalysis". J Am Chem Soc. 132 (18): 6507–6516. doi:10.1021/ja100974t. PMID 20397725. Retrieved 6 March 2013.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ Banks, R. D.; Blake, C. C. F.; Evans, P. R.; Haser, R.; Rice, D. W.; Hardy, G. W.; Merrett, M.; Phillips, A. W. (28 June 1979). "Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme". Nature. 279 (5716): 773–777. doi:10.1038/279773a0. PMID 450128.
- ^ "Phosphoglycerate kinase". Mechanism, Annotation and Classification In Enzymes (MACiE). Retrieved 4 March 2013.
- ^ Larsson-Raźnikiewicz M (January 1967). "Kinetic studies on the reaction catalyzed by phosphoglycerate kinase. II. The kinetic relationships between 3-phosphoglycerate, MgATP2-and activating metal ion". Biochim. Biophys. Acta. 132 (1): 33–40. doi:10.1016/0005-2744(67)90189-1. PMID 6030358.
{{cite journal}}: CS1 maint: date and year (link) - ^ Larsson-Raźnikiewicz, Märtha; Wiksell, Eva (1 March 1978). "Inhibition of phosphoglycerate kinase by salicylates". Biochimica et Biophysica Acta (BBA) - Enzymology. 523 (1): 94–100. doi:10.1016/0005-2744(78)90012-8. PMID 343818.
- ^ Yoshida A, Tani K (1983). "Phosphoglycerate kinase abnormalities: functional, structural and genomic aspects". Biomed. Biochim. Acta. 42 (11–12): S263–7. PMID 6689547.
- ^ a b c Beutler E (January 2007). "PGK deficiency". Br. J. Haematol. 136 (1): 3–11. doi:10.1111/j.1365-2141.2006.06351.x. PMID 17222195.
{{cite journal}}: CS1 maint: date and year (link) - ^ Rhodes, Melissa; Ashford, Linda; Manes, Becky; Calder, Cassie; Domm, Jennifer; Frangoul, Haydar (1 February 2011). "Bone marrow transplantation in phosphoglycerate kinase (PGK) deficiency". British Journal of Haematology. 152 (4): 500–502. doi:10.1111/j.1365-2141.2010.08474.x. PMID 21223252.
- ^ Zieker, Derek; Kã¶Nigsrainer, Ingmar; Tritschler, Isabel; Lã¶Ffler, Markus; Beckert, Stefan; Traub, Frank; Nieselt, Kay; Bã¼Hler, Sarah; Weller, Michael; Gaedcke, Jochen; Taichman, Russell S.; Northoff, Hinnak; Brã¼Cher, Björn L.D.M.; Kã¶Nigsrainer, Alfred (1). "Phosphoglycerate kinase 1 a promoting enzyme for peritoneal dissemination in gastric cancer". International Journal of Cancer. 126 (6): 1513–1520. doi:10.1002/ijc.24835. PMC 2811232. PMID 19688824.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ Lay, Angelina J.; Jiang, Xing-Mai; Kisker, Oliver; Flynn, Evelyn; Underwood, Anne; Condron, Rosemary; Hogg, Philip J. (14 December 2000). "Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase". Nature. 408 (6814): 869–873. doi:10.1038/35048596. PMID 11130727.
External links[edit]
- . GPnotebook https://www.gpnotebook.co.uk/simplepage.cfm?ID=-858783685.
{{cite web}}: Missing or empty|title=(help) - Phosphoglycerate+kinase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Illustration at arizona.edu