User:Elleevikram/sandbox
Options[edit]
Astressin-B
N-Acetylglutamic acid (switching articles ...)[edit]
Amino acid activation (does not cite any sources!)
Isobutanol (does not mention pathway but is still a long article)
I'm not sure how much more there is to add to cystine knot. The others seem fine. Isobutanol would have diagrams of the pathways and maybe simplification of section, but I'm not sure if its enough. - Dr. Tienson-Tseng
Editing Isobutanol[edit]
The primary drawback of E. coli is that it is susceptible to bacteriophages when being grown. This susceptibility could potentially shut down entire bioreactors. Furthermore, the native reaction pathway for isobutanol in E. coli functions optimally at a limited concentration of isobutanol in the cell. To reduce the sensitivity of E. coli to high concentrations, mutants of the enzymes involved in synthesis can be generated by random mutagenesis. By chance, some mutants may prove to be more tolerant of isobutanol which will enhance the overall yield of the synthesis. [1]
Bibliography (N-Acetylglutamic acid)[edit]
[2] (N-Acetylglutamic acid: an extracellular nod signal of Rhizobium trifolii ANU843 that induces root hair branching and nodule-like primordia in white clover roots.)[edit]
[3] (N-acetylglutamate and its changing role through evolution.)[edit]
[4] (Glutamate, at the interface between amino acid and carbohydrate metabolism.)[edit]
[5] (Conformational dynamics play important roles upon the function of N-acetylglutamate kinase.)[edit]
[6] (N-acetyl-L-glutamic acid.)[edit]
[7] (N-acetyl-glutamic acid: evaluation of acute and 28-day repeated dose oral toxicity and genotoxicity.)[edit]
[8] (Changes in N-acetylglutamate are involved in regulating urea synthesis in rats given a low gluten diet supplemented with L-lysine, L-methinone and L-threonine.)[edit]
[9] (N-acetylglutamate and N-acetylaspartate in soybeans (Glycine max L.), maize (Zea mays L.), [corrected] and other foodstuffs.)[edit]
[10] (The course of phosphorus in the reaction of N-acetyl-L-glutamate kinase)[edit]
[11] (purine, pyrimidine, etc)[edit]
[14] pubchem
Formatting Ideas (N-Acetylglutamic Acid)[edit]
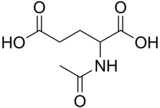
| |
| Names | |
|---|---|
| IUPAC name
2-Acetamidopentanedioic acid[15]
| |
| Other names
Acetylglutamic acid[citation needed]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| Abbreviations |
|
| 1727473 S | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
| MeSH | N-acetylglutamate |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C7H11NO5 | |
| Molar mass | 189.167 g·mol−1 |
| Appearance | White crystals |
| Density | 1 g mL−1 |
| Melting point | 191 to 194 °C (376 to 381 °F; 464 to 467 K) |
| 36 g L−1 | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
>7 g kg−1 (oral, rat) |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
N-Acetylglutamic acid (also referred to as N-Acetylglutamate, abbreviated NAG, chemical formula C7H11NO5) is biosynthesized from glutamic acid and acetyl-CoA by the enzyme N-acetylglutamate synthase. The reverse reaction, hydrolysis of the acetyl group, is catalyzed by a specific hydrolase. It is the first intermediate involved in the biosynthesis of arginine in prokaryotes and simple eukaryotes and a regulator in the process known as the urea cycle that converts toxic ammonia to urea for excretion from the body in vertebrates.
Discovery[edit]
N-Acetylglutamic acid is an extracellular metabolite isolated from the prokaryote Rhizobium trifolii that was characterized using many structure determination techniques such as proton nuclear magnetic resonance (H1 NMR) spectroscopy, Fourier-transform infrared spectroscopy, and gas chromatography-mass spectrometry.[16]
In Rhizobium, extracellular build-up of N-Acetylglutamic acid is due to metabolism involving nod factor genes on a symbiotic plasmid. When the nod factors are mutated, less N-Acetylglutamic acid is produced.
Biosynthesis[edit]
Prokaryotes and Simple Eukaryotes[edit]
In prokaryotes and simple eukaryotes, N-Acetylglutamic acid can be produced by N-acetylglutamate synthase (NAGS) or ornithine acetyltransferase (OAT).
Ornithine Acetyltransferase (OAT) Synthesis[edit]
OAT synthesizes N-Acetylglutamic acid from glutamate and acetylornithine and is the method of choice for production in prokaryotes that have the ability to synthesize the compound ornithine. [17]
N-Acetylglutamate Synthase (NAGS) Synthesis[edit]
N-Acetylglutamate synthase is an enzyme that serves as a replenisher of N-Acetylglutamic acid to supplement any N-Acetylglutamic acid lost by the cell through mitosis or degradation. NAGS synthesizes N-Acetylglutamic acid by catalyzing the addition of an acetyl group from acetyl-coenzyme A to glutamate. In prokaryotes with non-cyclic ornithine production, NAGS is the sole method of N-Acetylglutamic acid synthesis and is inhibited by arginine.[17] Acetylation of glutamate is thought to prevent glutamate from being used by proline biosynthesis.[18]
Vertebrates[edit]
Vertebrates do not have OAT therefore N-Acetylglutamic acid is exclusively synthesized by NAGS in liver mitochondria.[19] In contrast to prokaryotes, NAGS in mammals is enhanced by arginine, along with protamines. It is inhibited by N-Acetylglutamic acid and its analogues (other N-Acetyl compounds).[17]
Biological Roles[edit]
Vertebrate and Mammals[edit]
In vertebrae and mammals, N-Acetylglutamic acid is the the allosteric activator molecule to mitochondrial carbamyl phosphate synthetase I (CPSI) which is the first enzyme in the urea cycle.[20] It triggers the production of the first urea cycle intermediate, carbamyl phosphate. CPSI is inactive when N-Acetylglutamic acid is not present. In the liver and small intestines, N-Acetylglutamic acid-dependent CPSI produces citrulline, the second intermediate in the urea cycle. Liver cell distribution of N-Acetylglutamic acid is highest in the mitochondria at 56% of total N-Acetylglutamic acid availability, 24% in the nucleus, and the remaining 20% in the cytosol. Aminoacylase I in liver and kidney cells degrades N-Acetylglutamic acid to glutamate and acetate.[21] In contrast, N-Acetylglutamic acid is not the allosteric cofactor to carbamyl phosphate synthetase found in the cytoplasm, which is involved in pyrimidine synthesis.[22]
N-Acetylglutamic acid concentrations increase when protein consumption increases due to the accumulation of ammonia that must be secreted through the urea cycle, which supports the role of N-Acetylglutamic acid as the cofactor for CPSI. Furthermore, N-Acetylglutamic acid can be found in many commonly consumed foods such as soybean, corn, coffee, etc., with cocoa powder containing a notably high concentration.[23]
Deficiency in N-Acetylglutamic acid in humans is an autosomal recessive disorder that results in blockage of urea production which ultimately increases the concentration of ammonia in the blood (hyperammonemia). Deficiency can be caused by defects in the NAGS coding gene or by deficiencies in the precursors essential for synthesis.[17]
Bacteria[edit]
N-Acetylglutamic acid is the second intermediate in the arginine production pathway in Escherichia coli (E. coli) and is produced via NAGS.[18] In this pathway, N-Acetylglutamic acid kinase (NAGK) catalyzes the phosphorylation of the gamma (third) carboxyl group of N-Acetylglutamic acid using the energy released by the hydrolysis of a phosphate group from an adenosine triphosphate (ATP) molecule.[24]
White clover seedling roots[edit]
Rhizobium can form a symbiotic relationship with white clover seedling roots and form colonies. The extracellular N-Acetylglutamic acid produced by these bacteria have three morphological effects on the white clover seedling roots: branching of root hairs, swelling of root tips, and increase in the number of cell divisions in undifferentiated cells found on the outer-most cell layer of the root. This suggests that N-Acetylglutamic acid is involved in the stimulation of mitosis. The same effects were observed on the strawberry clover, but not in legumes. The effects of N-Acetylglutamic acid on the clover species were more potent than the effects from glutamine, glutamate, arginine, or ammonia.[17]
Structure[edit]
Proton (H1) NMR Spectroscopy[edit]

The molecular structure of N-Acetylglutamic acid was determined using proton H1 NMR spectroscopy.[16] Proton NMR reveals the presence and functional group location of protons based on chemical shifts recorded on the spectrum (shown right).

The hydrogens that are read by the spectrophotometer are explicitly shown in the image on the left.
Carbon (C12) NMR Spectroscopy[edit]

Like H1 NMR, carbon (C12) NMR spectroscopy is a method used in molecular structure determination. Carbon NMR reveals the types of carbons present in a molecule based on chemical shifts that correspond to certain functional groups. The C12 NMR spectrum of N-Acetylglutamic acid is shown on the right.
Notes & Reminders[edit]
***applications of nag??
chemistry section??
add citations for info found in the chembox
toxin levels??? from [7]
Bibliography (Astressin-B)[edit]
[25] (Corticotropin-releasing factor peptide antagonists: design, characterization and potential clinical relevance)[edit]
Notes:
- Astressin B is an antagonist to corticotropin releasing factor receptor type 1 (CRFR1) and corticotropin releasing factor type 2 (CRFR2). It could serve as treatment for irritable bowel syndrome
- Astressin B removes the negative affects of ghrelin on luteinizing hormone in Rhesis monkeys that have had their ovaries removed
[26] (CRF Receptor Antagonist Astressin-B Reverses and Prevents Alopecia in CRF Over-Expressing Mice)[edit]
Notes:
- Corticotropin-releasing factor (CRF) is important in the stress response in many organisms (such as humans and mice)
- Over-expression of CRF causes chronic stress which can cause alopecia
- Injecting astressin B into a body cavity or under the skin was found to reverse hair loss
- 50-90% of hair lost was regained in about a month
- Visceral fat amount (side effect of over-expressed CRF) was unaffected by astressin B injection
- Astressin B was synthesized by the Peptide Biology Laboratory at the Salk Institute in La Jolla, CA
- Hair follicle was revived by astressin B from telogen to anagen phase
[27] (UCLA Newsroom. Regrowing hair: UCLA-VA researchers may have accidentally discovered a solution)
- This experiment was conducted jointly by University of California, Los Angeles, the Veterans Administration, and the Salk Institute
[28](Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration)[edit]
Notes:
- Rhesus monkeys exhibit social subordination leading to psychosocial stress. CRF induces increased sensitivity to ghrelin, which is a peptide that increases appetite. Astressin B treatment to socially subordinate monkeys improved their previously low appetite that was due to high cortisol levels. Astressin B decreases cortisol levels by antagonizing CRF receptor 1 and 2
- This experiment involved treatment of dominant and subordinate Rhesus monkeys with astressin B.
- Dispersal of astressin B increased positive association among dominant monkey groups. Subordinate females did not exhibit any change in affiliative behavior
[29] (The effect of Nesfatin-1 on food intake in neonatal chicks: role of CRF1 /CRF2 and H1/ H3 receptors.)[edit]
Notes:
- Experiment to test Nesfatin-1 effects on food consumption by neonatal chicks.
- Nesfatin-1 decreased appetite while co-injection of Nesfatin-1 and Astressin B inhibited the effects of Nesfatin-1 alone
[edit]
Notes:
- Another type of Astressin B is Astressin 2-B which only inhibits CRFR2
- Astressin 2-B in high dosage reduced anxiety because they inhibit the CRFR2 in the medial amygdala in male Wistar rats
- A low dosage of Astressin 2-B did not reduce anxiety on its own but was able to counter the effects of administration of anxiety-inducing urocortin 2
Formatting Ideas (Astressin-B)[edit]
Include section on experiments with mice, then another on the Rhesus monkeys
 | This is a user sandbox of Elleevikram. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
- ^ Chong, Huiqing; Geng, Hefang; Zhang, Hongfang; Song, Hao; Huang, Lei; Jiang, Rongrong (2013-11-06). "EnhancingE. coliisobutanol tolerance through engineering its global transcription factor cAMP receptor protein (CRP)". Biotechnology and Bioengineering. 111 (4): 700–708. doi:10.1002/bit.25134. ISSN 0006-3592.
- ^ Philip-Hollingsworth, S.; Hollingsworth, R. I.; Dazzo, F. B. (1991-09-05). "N-Acetylglutamic acid: an extracellular nod signal of Rhizobium trifolii ANU843 that induces root hair branching and nodule-like primordia in white clover roots". Journal of Biological Chemistry. 266 (25): 16854–16858. ISSN 0021-9258. PMID 1885611.
- ^ Caldovic, Ljubica; Tuchman, Mendel (2003-06-01). "N-acetylglutamate and its changing role through evolution". The Biochemical Journal. 372 (Pt 2): 279–290. doi:10.1042/BJ20030002. ISSN 0264-6021. PMC 1223426. PMID 12633501.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Brosnan, J. T. (April 2000). "Glutamate, at the interface between amino acid and carbohydrate metabolism". The Journal of Nutrition. 130 (4S Suppl): 988S–90S. ISSN 0022-3166. PMID 10736367.
- ^ Yang, Xiaorong (May 2017). "Conformational dynamics play important roles upon the function of N-acetylglutamate kinase". Applied Microbiology and Biotechnology. 101 (9): 3485–3492. doi:10.1007/s00253-017-8237-1. ISSN 1432-0614. PMID 28341883.
- ^ Dobson, A. J.; Gerkin, R. E. (1997-01-15). "N-acetyl-L-glutamic acid". Acta Crystallographica. Section C, Crystal Structure Communications. 53 ( Pt 1): 73–76. ISSN 0108-2701. PMID 9037750.
- ^ Harper, Marc S.; Amanda Shen, Z.; Barnett, John F.; Krsmanovic, Ljubica; Myhre, Abby; Delaney, Bryan (November 2009). "N-acetyl-glutamic acid: evaluation of acute and 28-day repeated dose oral toxicity and genotoxicity". Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 47 (11): 2723–2729. doi:10.1016/j.fct.2009.07.036. ISSN 1873-6351. PMID 19654033.
- ^ Tujioka, Kazuyo; Tuchiya, Tamami; Shi, Xianglan; Ohsumi, Miho; Hayase, Kazutoshi; Yokogoshi, Hidehiko (2009). "Changes in N-acetylglutamate are involved in regulating urea synthesis in rats given a low gluten diet supplemented with L-lysine, L-methinone and L-threonine". Journal of Nutritional Science and Vitaminology. 55 (5): 417–422. ISSN 1881-7742. PMID 19926928.
- ^ Hession, Aideen O.; Esrey, Elizabeth G.; Croes, Robert A.; Maxwell, Carl A. (2008-10-08). "N-acetylglutamate and N-acetylaspartate in soybeans (Glycine max L.), maize (Zea mays L.), [corrected] and other foodstuffs". Journal of Agricultural and Food Chemistry. 56 (19): 9121–9126. doi:10.1021/jf801523c. ISSN 1520-5118. PMID 18781757.
- ^ Gil-Ortiz, Fernando; Ramón-Maiques, Santiago; Fita, Ignacio; Rubio, Vicente (2003-08-01). "The course of phosphorus in the reaction of N-acetyl-L-glutamate kinase, determined from the structures of crystalline complexes, including a complex with an AlF(4)(-) transition state mimic". Journal of Molecular Biology. 331 (1): 231–244. ISSN 0022-2836. PMID 12875848.
- ^ Pelley, John W. (2007). Elsevier's Integrated Biochemistry. Elsevier. pp. 117–122. doi:10.1016/b978-0-323-03410-4.50020-1. ISBN 9780323034104.
- ^ "Predict 1H proton NMR spectra". www.nmrdb.org. Retrieved 2018-05-14.
- ^ "Predict 13C carbon NMR spectra". www.nmrdb.org. Retrieved 2018-05-14.
- ^ Pubchem. "Acetyl glutamic acid". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-05-14.
- ^ "N-Acetyl-DL-glutamic acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 25 March 2005. Identification. Retrieved 25 June 2012.
- ^ a b Philip-Hollingsworth S, Hollingsworth RI, Dazzo FB (September 1991). "N-Acetylglutamic acid: an extracellular nod signal of Rhizobium trifolii ANU843 that induces root hair branching and nodule-like primordia in white clover roots". The Journal of Biological Chemistry. 266 (25): 16854–8. PMID 1885611.
- ^ a b c d e Caldovic L, Tuchman M (June 2003). "N-acetylglutamate and its changing role through evolution". The Biochemical Journal. 372 (Pt 2): 279–90. doi:10.1042/BJ20030002. PMC 1223426. PMID 12633501.
- ^ a b Caldara M, Dupont G, Leroy F, Goldbeter A, De Vuyst L, Cunin R (March 2008). "Arginine biosynthesis in Escherichia coli: experimental perturbation and mathematical modeling". The Journal of Biological Chemistry. 283 (10): 6347–58. doi:10.1074/jbc.M705884200. PMID 18165237.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Brosnan JT (April 2000). "Glutamate, at the interface between amino acid and carbohydrate metabolism". The Journal of Nutrition. 130 (4S Suppl): 988S–90S. doi:10.1093/jn/130.4.988S. PMID 10736367.
- ^ Auditore, Joseph V.; Wade, Littleton; Olson, Erik J. (1966-11). "OCCURRENCE OF N-ACETYL-l-GLUTAMIC ACID IN THE HUMAN BRAIN". Journal of Neurochemistry. 13 (11): 1149–1155. doi:10.1111/j.1471-4159.1966.tb04272.x. ISSN 0022-3042.
{{cite journal}}: Check date values in:|date=(help) - ^ Harper MS, Amanda Shen Z, Barnett JF, Krsmanovic L, Myhre A, Delaney B (November 2009). "N-acetyl-glutamic acid: evaluation of acute and 28-day repeated dose oral toxicity and genotoxicity". Food and Chemical Toxicology. 47 (11): 2723–9. doi:10.1016/j.fct.2009.07.036. PMID 19654033.
- ^ Pelley JW (2007). "Chapter 14: Purine, Pyrimidine, and Single-Carbon Metabolism". Elsevier's Integrated Biochemistry. Elsevier. pp. 117–122. doi:10.1016/b978-0-323-03410-4.50020-1. ISBN 978-0-323-03410-4.
- ^ Hession AO, Esrey EG, Croes RA, Maxwell CA (October 2008). "N-acetylglutamate and N-acetylaspartate in soybeans (Glycine max L.), maize (Zea mays L.), [corrected] and other foodstuffs". Journal of Agricultural and Food Chemistry. 56 (19): 9121–6. doi:10.1021/jf801523c. PMID 18781757.
- ^ Gil-Ortiz F, Ramón-Maiques S, Fita I, Rubio V (August 2003). "The course of phosphorus in the reaction of N-acetyl-L-glutamate kinase, determined from the structures of crystalline complexes, including a complex with an AlF(4)(-) transition state mimic". Journal of Molecular Biology. 331 (1): 231–44. PMID 12875848.
- ^ Rivier, Jean E.; Rivier, Catherine L. (2014-4). "Corticotropin-releasing factor peptide antagonists: design, characterization and potential clinical relevance". Frontiers in neuroendocrinology. 35 (2): 161–170. doi:10.1016/j.yfrne.2013.10.006. ISSN 0091-3022. PMC 3965584. PMID 24269930.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Wang, Lixin; Million, Mulugeta; Rivier, Jean; Rivier, Catherine; Craft, Noah; Stenzel-Poore, Mary P.; Taché, Yvette (2011-02-16). "CRF Receptor Antagonist Astressin-B Reverses and Prevents Alopecia in CRF Over-Expressing Mice". PLOS ONE. 6 (2): e16377. doi:10.1371/journal.pone.0016377. ISSN 1932-6203. PMC 3040186. PMID 21359208.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Rivero, Enrique. "Regrowing hair: UCLA-VA researchers may have accidentally discovered a solution". UCLA Newsroom. Retrieved 2018-05-03.
- ^ Michopoulos, Vasiliki; Loucks, Tammy; Berga, Sarah L.; Rivier, Jean; Wilson, Mark E. (2010-10-01). "Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration". Endocrine. 38 (2): 227–234. doi:10.1007/s12020-010-9378-5. ISSN 1355-008X.
- ^ Heidarzadeh, Hooman; Zendehdel, Morteza; Babapour, Vahab; Gilanpour, Hasan (March 2018). "The effect of Nesfatin-1 on food intake in neonatal chicks: role of CRF1 /CRF2 and H1/ H3 receptors". Veterinary Research Communications. 42 (1): 39–47. doi:10.1007/s11259-017-9706-9. ISSN 1573-7446. PMID 29280084.
- ^ Alves, Stephanie W. E.; Portela, Natasha C.; Silva, Mariana S.; Céspedes, Isabel C.; Bittencourt, Jackson C.; Viana, Milena B. (2016-05-15). "The activation and blockage of CRF type 2 receptors of the medial amygdala alter elevated T-maze inhibitory avoidance, an anxiety-related response". Behavioural Brain Research. 305: 191–197. doi:10.1016/j.bbr.2016.03.013. ISSN 1872-7549. PMID 26965566.
