User:GM1000/sandbox
Lactic acid is an acid that is very common in nature. Carl Wilhelm Scheele discovered it in milk that had gone sour, in 1780.[1] Since then, it has been used in various applications, and it is involved in anaerobic glycolysis by giving the muscles energy to continue working. It does not cause the pain in muscles when exercising contrary to popular belief.[2]

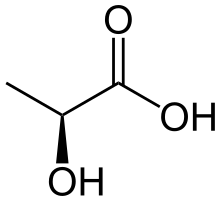
Structure[edit]
Lactic Acid is a carboxylic acid with a chemical formula of C3H6O3. Its molecular weight is 90.078 g/mol. According to CAMEO chemicals, "Lactic acid is colorless to yellow odorless syrupy liquid."[3]
Role in Anaerobic Glycolysis[edit]
Many people think that lactic acid causes pain in the muscles during exercise, but this compound actually acts as an energy source. When exercising, the body first uses aerobic glycolysis to get energy which consumes oxygen. When there is no longer oxygen, or very little of it, the muscles start using anaerobic glycolysis which does not need oxygen. In this process, muscles break down carbohydrates which produces energy, but creates the byproduct pyruvate. At this point, if a supply of oxygen is reestablished to the muscles, pyruvate is broken down generating energy, but if there is still insufficient oxygen, pyruvate is converted into lactic acid. Lactic acid is then dissociated into lactate and a hydrogen ion.[4] Lactate is metabolized in the body producing energy, but at some point lactate levels may rise if the body cannot get rid of the lactate fast enough. As a result, the body starts feeling fatigued, but it is not because of the buildup of lactate; it is because of the buildup of hydrogen ions in the muscles from the lactic acid. [5]
Usage[edit]
Lactic acid exists in many food products due to fermentation.[6] In meats, it is used to prevent spoiling and to kill bacteria. It can also regulate the acidity in beverages and prevent pickled vegetables from going bad. It can enhance the flavor of foods such as dairy and bread. Lactic acid is also used in non-food products. In medicine, it acts as a pH-regulator for pharmaceuticals, and it is a part of medical devices.[7] Its properties are utilized to achieve certain outcomes such as biodegradability and acidity. Finally, lactic acid is a part of animal feed because it benefits the animal's health.
Role in Cancer[edit]
Lactic acid has been used in cancer treatment. Some the most aggressive and resistant to treatment cells are hypoxic, low oxygen, cells. These cells need to burn up a lot of glucose to stay alive because they are not near blood vessels to get oxygen. Tumor cells near blood vessels with access to oxygen in turn prefer to burn lactate so that hypoxic cells can burn glucose to stay alive. Researchers from Duke University Medical Center and the Université Catholique de Louvain (UCL) have found in experiments that when lactate is cut off from tumor cells, the cells near blood vessels with access to oxygen start to burn glucose causing hypoxic cells to not have any energy sources and die. This approach may be used in future treatments for cancer because currently hypoxic cells are very hard to kill with chemotherapy.[8]
- ^ "LACTIC ACID - history". Lactic Acid. Retrieved 2018-02-17.
- ^ Miller, Joe. "Muscle Fatigue & Soreness from Lactic Acid". LiveStrong. Retrieved 20 February 2018.
- ^ "Lactic Acid". PubChem. Retrieved 2018-02-12.
- ^ "Lactic Acid - lactic acid & lactate". Lactic Acid. Retrieved 2018-02-17.
- ^ Miller, Joe. "Muscle Fatigue & Soreness from Lactic Acid". LiveStrong. Retrieved 20 February 2018.
- ^ "Lactic Acid - In Food". Lactic Acid. Retrieved 2018-02-17.
- ^ "Lactic Acid - In Non-Food". Lactic Acid. Retrieved 2018-02-17.
- ^ "Lactic Acid Found To Fuel Tumors". ScienceDaily. Retrieved 2018-02-15.

