User:Khrisfaiss/sandbox
Spinal Cord Stimulator Sandbox
| Khrisfaiss/sandbox | |
|---|---|
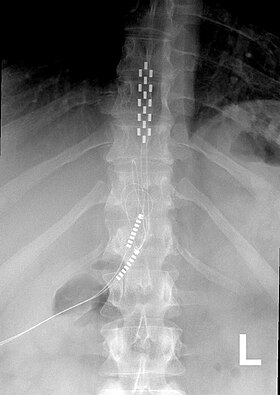 Anterior view X-ray of a spinal cord stimulator (SCS) implanted in the thoracic spine |
A Spinal Cord Stimulator (SCS) or Dorsal Column Stimulator (DCS) is a type of implantable neuromodulation device (sometimes called a "pain pacemaker") that is used to send electrical signals to select areas of the spinal cord (dorsal columns) for the treatment of certain pain conditions. SCS has provided therapeutic benefits for otherwise treatment resistant pain disorders. However, SCS is not effective for all types of pain, and may not be effective for every patient, thus SCS is considered for patients who have chronic pain that have failed conservative therapies.
In the United States Failed Back Surgery Syndrome is the most common indication while in Europe the most common indication is peripheral ischemia.[1]
Currently the Food and Drug Administration (FDA) has approved SCS as a treatment for Failed Back Surgery Syndrome (FBSS), chronic pain, and pain in the extremities from nerve damage.[2]
Medical uses[edit]
Failed Back Surgery Syndrome[edit]
FBSS, classified as mixed pain syndrome (neuropathic and nociceptive), is persistent or recurrent pain mainly involving the lower back and/or legs after successful spinal surgery. It affects about 40% of patients who undergo spinal surgeries. Several studies have shown that SCS is effective for FBSS.[3][4][5][6]
Chronic Pain[edit]
Pain in the extremities from nerve damage[edit]
SCS is also indicated in the treatment of inoperable ischemic limb pain.[7] It can also modulate the function of the sympathetic nervous system and increase norepinephrine release in refractory angina pectoris, [8]decreasing the probability of angina attack. SCS has also been used to treat patients with frequent migraines, using electrodes implanted in the bilateral suboccipital region. [9]
Mechanism and Technical Consideration[edit]
The mechanism by which current applied to the spinal cord elicits the action of pain relief and paresthesia is not well understood, but it is theorized that it is multifactorial. The initial theory behind the analgesia provided from SCS comes from Gate Control Theory, but the pain relief is now considered to be more complex.[10]
While there are new advances in the types of neuromodulation, the objective of traditional SCS therapy is to "mask" or replace pain sensation with paresthesia or comfortable tingling by altering the pain processing of the Central Nervous System. In order to do this, a technician or device representative must map the area of stimulation to the areas of nociceptive or neuropathic pain. Linderoth and others[11] [12] have noted that the mechanism of analgesia when SCS is applied in neuropathic pain states may be very different from that involved in analgesia due to limb ischemia. Additionally, patients may report the paresthesias as unpleasant. [13][14]
Patient Selection[edit]
Each patient must be screened to determine candidacy for this treatment. Screening of individuals includes evaluation for a medically indicated diagnosis as well as a history and physical examination to rule out medical conditions that would increase the risk for failure or complications. Often a psychiatric evaluation may be performed to determine appropriate candidates as well.
Spinal Cord Stimulation Trial[edit]
Once a patient is evaluated and deemed an appropriate candidate for SCS, a temporary implant is placed, called a trial, to evaluate if the treatment will be beneficial. The procedure proceeds with the patient under light sedation and are assessed to be wide awake and responsive to commands throughout the entire procedure.
The placement technique traditionally involves placing two percutaneous leads in the epidural space of the spinal cord at a site lower than the desired position, often beginning at the L1-L2 spinal level. Access to the epidural space is gained with an angulated needle and the leads are then guided into the epidural space and advanced to the desired position. [15] Once the leads are placed, a technician maps the patients sensation of paresthesias and recommends minor adjustments to the SCS placement to maximize overlap between paresthesias and the patient's pain.
Spinal Cord Stimulator Implant[edit]
Percutaneous
Paddle
Other Considerations[edit]
Burst
High Frequency
PMI: 27139915 Burst stimulation
Contraindications[edit]
Currently SCS is not an FDA approved treatment solely for the treatment of back pain without extremity pain. [15]
Contraindications are conditions or factors that suggest that a particular technique should not be used. In the case of spinal cord stimulation, the most concerning contraindications include coagulopathy[16], infection[17], pacemaker[18], imaging studies indicating difficulty in placement, or concerns that arise during psychological evaluation.
Risks/Complications[edit]
Complications with SCS range from simple easily correctable problems to devastating paralysis, nerve injury and death. However, in a 7-year follow-up, the overall complication rate was 5-18%. The most common complications involve hardware related issues including lead migration, lead breakage. Infection is also one of the most common complications.[19] Other complications include haematomas(subcutaneous or epidural), cerebrospinal fluid (CSF) leak, post dural puncture headache, discomfort at pulse generator site, seroma and transient paraplegia.
Hardware Related Complications[edit]
Lead Migration[edit]
The most common hardware related complication is lead migration, in which the implanted electrodes move from their original placement. With this complication, recapturing paraesthesia coverage can be attempted with reprogramming.[20] In circumstances involving major lead migration a reoperation may be required to reset the the lead placement.[21] Studies differ greatly in reporting the percentage of patients who have lead migration but the majority of studies report in the range of 10-25% of lead migration for spinal cord stimulation.[21]
Lead migration with a paddle technique is less likely to incur a migration compared to percutaneous leads.
Lead Fracture[edit]
Battery/Generator Failure[edit]
Biological Complications[edit]
[edit]
Wound Infection[edit]
Dural Puncture[edit]
Neurological Injury[edit]
Device Removal[edit]
Recovery or Rehabilitation[edit]
Patients are typically asked to return to clinic within one week of the implantation to ensure the wound is healing well, and to ensure there is no infection. In addition to ruling out surgical complications, the device may often need reprogramming during the first few post-operative follow ups.
History[edit]
Electrotherapy of pain by neurostimulation began shortly after Melzack and Wall proposed the gate control theory in 1965. This theory proposed that nerves carrying painful peripheral stimuli and nerves carrying touch and vibratory sensation both terminate in the dorsal horn (the gate) of spinal cord. It was hypothesized that input to the latter could be manipulated to “close the gate” to the former. As an application of the gate control theory, Shealy et al. implanted the first spinal cord stimulator device directly on the dorsal column for the treatment of chronic painand in 1971, Shimogi and colleagues first reported the analgesic properties of epidural spinal cord stimulation. Since then this technique has undergone numerous technical and clinical developments.
At this time neurostimulation for the treatment of pain is used with nerve stimulation, spinal cord stimulation, deep brain stimulation, and motor cortex stimulation.
Society and culture[edit]
Special populations[edit]
SCS is finding its way to be applied to Parkinson’s disease. Recently, a case study demonstrated the first successful result of this technique in a patient with Parkinson's disease. More complex and power efficient microprocessor based equipment increasing the battery life could be developed. Closed loop bio-feedback systems which communicate and record neural responses following spinal cord stimulation could be applied and utilized In the future, it might be possible to combine SCS with implanted drug delivery systems to produce synergistic effects minimizing side effects and complications. Strong evidence is still lacking for SCS which may emerge in near future following robust research studies complimenting the rapid technological advances that is taking place in the field of SCS.
Other animals[edit]
 | This is a user sandbox of Khrisfaiss. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Technical consideration[edit]
Equipment[edit]
SCS, in simplest form, consists of a pulse generator with its remote controls, implanted stimulating electrodes and conducting wires connecting the electrodes to the generator.[22]
Generator[edit]
The generator is implanted subcutaneously, It can be a complete pulse generator module (an "implantable pulse generator" or IPG) or simply a radio frequency (RF) receiver. The IPG contains a battery; this battery may be rechargeable using an external wireless power charger, so that it does not need to be replaced surgically when it loses charge. The RF receiver is driven by an external transmitter from which it gets its power and pulses. This external transmitter has a battery which can be easily replaced. RF receivers have traditionally been used for patients who require high power settings that would quickly deplete a primary-cell IPG.[22]
Remote Control
The patient is also provided with a remote control to turn on and off the stimulator. Depending on the device and the surgeon’s preference, it may also change the programming of the stimulation patterns. The surgeon has a programming device that could be used to modify a wide range of stimulation settings of the RF generator.[22]
Various current, voltage and waveform configurations are possible. Some stimulators vary voltage to achieve a constant current, while others vary current to achieve a constant voltage.[22]
Electrodes[edit]
The electrodes, which consist of an array of leads, can be percutaneous type or paddle type. Percutaneous electrodes are easier to insert than the paddle type, which require an incision over the spinal cord and a laminectomy.[23]

Insertion procedures and techniques[edit]
SCS procedure involves careful placement of electrodes in the epidural space, followed by a trial period of 5-7 days. If the pain relief is satisfactory, the electrodes are then anchored to the interspinal ligaments, the pulse generator is positioned and implanted, and the wires are connected. The stimulator is then programmed, and postoperative care performed.[22]
Selecting the level of stimulation[edit]
The representation of the dermatomal level in the dorsal columns of the spinal cord is much higher than the corresponding vertebral level. For instance, the sweet spot for sciatic pain (dermatomal level L5/S1) is around T10 nerve.[22] See dermatome and Spinal cord segments.
Electrodes selection[edit]
For the SCS to be effective, the area of paresthesia must overlap the area of pain. Selection of leads depends on which arrangement will give the best paresthesia coverage to the painful area.
Generator implant[edit]
The IPG or the RF unit is usually implanted in the lower abdominal area or in the posterior superior gluteal region. It should be in a location that patients can access with their dominant hand for adjustment of their settings with the patient-held remote control. The decision to use a fully implantable IPG or an RF unit depends on several considerations. If the patient’s pain pattern requires the use of many electrodes with high power settings, an RF unit should be used. The IPG battery life will largely depend on the power settings utilized, but the newer IPG units will generally last several years at average power settings.[22]

Programming[edit]
Programming involves selecting the electrode stimulating configuration, and adjusting the amplitude, width and frequency of electrical pulses. Amplitude (intensity of stimulation) is specified in milliamperes or volts depending on the system used. Lower amplitudes are used for peripheral nerves and paddle leads. Pulse width (the duration of each pulse) usually varies from 100 to 400 us, with wider pulses producing a broader area of paraesthesia. Pulse frequency (number of pulses per second) is usually between 20 and 120 hertz. It is an individual preference: some patients choose low frequency beating sensation whereas others prefer high frequency buzzing.[22]
Selection of lowest possible setting on all parameters is important in conserving battery life in non-rechargeable models of SCS. Cycling of stimulation is also employed to save battery life. Changing of stimulator program may have to be undertaken during the course of therapy and follow-up.[22]
Patient selection[edit]
Appropriate patients for neurostimulation implants must meet the following criteria: the patient has a diagnosis amenable to this therapy, the patient has failed conservative therapy, significant psychological issues have been ruled out, and a trial has demonstrated pain relief. A trial period of stimulation over a period of 5–7 days should follow the psychiatric evaluation to demonstrate its effectiveness. This part of the protocol is important because of the cost of the equipment and the invasive nature of the procedure. The trial is considered successful if the patient achieves more than a 50% reduction in pain.[23]
History[edit]
Electrotherapy of pain by neurostimulation began shortly after Melzack and Wall proposed the gate control theory in 1965.[24] This theory proposed that nerves carrying painful peripheral stimuli and nerves carrying touch and vibratory sensation both terminate in the dorsal horn (the gate) of spinal cord. It was hypothesized that input to the latter could be manipulated to “close the gate” to the former. As an application of the gate control theory, Shealy et al. implanted the first spinal cord stimulator device directly on the dorsal column for the treatment of chronic pain[25] and in 1971, Shimogi and colleagues first reported the analgesic properties of epidural spinal cord stimulation.[26] Since then this technique has undergone numerous technical and clinical developments.[23]
At this time neurostimulation for the treatment of pain is used with nerve stimulation, spinal cord stimulation, deep brain stimulation, and motor cortex stimulation.
Cost effectiveness[edit]
The cost effectiveness of spinal cord stimulation in the treatment of chronic back pain was evaluated by Kumar and colleagues in 2002.[27] They examined 104 patients with failed back surgery syndrome. Of the 104 patients, 60 were implanted with a spinal cord stimulator. Both groups were monitored over a period of five years. The stimulation group annual cost was $29,000 versus $38,000 in the other group. 15% returned to work in the stimulation group versus 0% in the other group. The higher costs in the nonstimulator group were in the categories of medications, emergency center visits, x-rays, and ongoing physician visits. See also[28] for another study of cost effectiveness. See[23] for list of such studies on cost effectiveness in various applications of SCS.
Research[edit]
SCS is finding its way to be applied to Parkinson’s disease.[29][30] Recently, a case study demonstrated the first successful result of this technique in a patient with Parkinson's disease.[31] More complex and power efficient microprocessor based equipment increasing the battery life could be developed. Closed loop bio-feedback systems which communicate and record neural responses following spinal cord stimulation could be applied and utilized[32] In the future, it might be possible to combine SCS with implanted drug delivery systems to produce synergistic effects minimizing side effects and complications. Strong evidence is still lacking for SCS which may emerge in near future following robust research studies complimenting the rapid technological advances that is taking place in the field of SCS.[22]
- ^ Eldabe, Sam; Kumar, Krishna; Buchser, Eric; Taylor, Rod S. (July 2010). "An analysis of the components of pain, function, and health-related quality of life in patients with failed back surgery syndrome treated with spinal cord stimulation or conventional medical management". Neuromodulation: Journal of the International Neuromodulation Society. 13 (3): 201–209. doi:10.1111/j.1525-1403.2009.00271.x. ISSN 1525-1403. PMID 21992833.
- ^ Song, Jason J.; Popescu, Adrian; Bell, Russell L. (May 2014). "Present and potential use of spinal cord stimulation to control chronic pain". Pain Physician. 17 (3): 235–246. ISSN 2150-1149. PMID 24850105.
- ^ Kumar, Krishna; Taylor, Rod S.; Jacques, Line; Eldabe, Sam; Meglio, Mario; Molet, Joan; Thomson, Simon; O'Callaghan, Jim; Eisenberg, Elon (October 2008). "The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation". Neurosurgery. 63 (4): 762–770, discussion 770. doi:10.1227/01.NEU.0000325731.46702.D9. ISSN 1524-4040. PMID 18981888.
- ^ North, R. B.; Kidd, D. H.; Piantadosi, S. (1995). "Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design". Acta Neurochirurgica. Supplement. 64: 106–108. ISSN 0065-1419. PMID 8748595.
- ^ Barolat, G.; Oakley, J. C.; Law, J. D.; North, R. B.; Ketcik, B.; Sharan, A. (April 2001). "Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain". Neuromodulation: Journal of the International Neuromodulation Society. 4 (2): 59–66. doi:10.1046/j.1525-1403.2001.00059.x. ISSN 1094-7159. PMID 22151612.
- ^ Turner, J. A.; Loeser, J. D.; Bell, K. G. (December 1995). "Spinal cord stimulation for chronic low back pain: a systematic literature synthesis". Neurosurgery. 37 (6): 1088–1095, discussion 1095–1096. ISSN 0148-396X. PMID 8584149.
- ^ Amann, W.; Berg, P.; Gersbach, P.; Gamain, J.; Raphael, J. H.; Ubbink, D. Th; European Peripheral Vascular Disease Outcome Study SCS-EPOS (September 2003). "Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS)". European Journal of Vascular and Endovascular Surgery: The Official Journal of the European Society for Vascular Surgery. 26 (3): 280–286. ISSN 1078-5884. PMID 14509891.
- ^ Taylor, Rod S.; De Vries, Jessica; Buchser, Eric; Dejongste, Mike J. L. (2009-03-25). "Spinal cord stimulation in the treatment of refractory angina: systematic review and meta-analysis of randomised controlled trials". BMC cardiovascular disorders. 9: 13. doi:10.1186/1471-2261-9-13. ISSN 1471-2261. PMC 2667170. PMID 19320999.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Matharu, Manjit S.; Bartsch, Thorsten; Ward, Nick; Frackowiak, Richard S. J.; Weiner, Richard; Goadsby, Peter J. (January 2004). "Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study". Brain: A Journal of Neurology. 127 (Pt 1): 220–230. doi:10.1093/brain/awh022. ISSN 0006-8950. PMID 14607792.
- ^ Melzack, R.; Wall, P. D. (1965-11-19). "Pain mechanisms: a new theory". Science (New York, N.Y.). 150 (3699): 971–979. ISSN 0036-8075. PMID 5320816.
- ^ Linderoth, B.; Foreman, R. D. (July 1999). "Physiology of spinal cord stimulation: review and update". Neuromodulation: Journal of the International Neuromodulation Society. 2 (3): 150–164. doi:10.1046/j.1525-1403.1999.00150.x. ISSN 1094-7159. PMID 22151202.
- ^ Oakley, John C.; Prager, Joshua P. (2002-11-15). "Spinal cord stimulation: mechanisms of action". Spine. 27 (22): 2574–2583. doi:10.1097/01.BRS.0000032131.08916.24. ISSN 1528-1159. PMID 12435996.
- ^ Deer, Timothy R.; Krames, Elliot; Mekhail, Nagy; Pope, Jason; Leong, Michael; Stanton-Hicks, Michael; Golovac, Stan; Kapural, Leo; Alo, Ken (August 2014). "The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee". Neuromodulation: Journal of the International Neuromodulation Society. 17 (6): 599–615, discussion 615. doi:10.1111/ner.12204. ISSN 1525-1403. PMID 25112892.
- ^ Sinclair, Chantelle; Verrills, Paul; Barnard, Adele (2016-07-01). "A review of spinal cord stimulation systems for chronic pain". Journal of Pain Research. Volume 9: 481–492. doi:10.2147/jpr.s108884.
{{cite journal}}:|volume=has extra text (help)CS1 maint: unflagged free DOI (link) - ^ a b Patel, Vikram B.; Wasserman, Ronald; Imani, Farnad (2015-08-22). "Interventional Therapies for Chronic Low Back Pain: A Focused Review (Efficacy and Outcomes)". Anesthesiology and Pain Medicine. 5 (4). doi:10.5812/aapm.29716. ISSN 2228-7523. PMC 4604560. PMID 26484298.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Narouze, Samer; Benzon, Honorio T.; Provenzano, David A.; Buvanendran, Asokumar; De Andres, José; Deer, Timothy R.; Rauck, Richard; Huntoon, Marc A. (May 2015). "Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain". Regional Anesthesia and Pain Medicine. 40 (3): 182–212. doi:10.1097/AAP.0000000000000223. ISSN 1532-8651. PMID 25899949.
- ^ Knezevic, Nebojsa N.; Candido, Kenneth D.; Rana, Shalini; Knezevic, Ivana (July 2015). "The Use of Spinal Cord Neuromodulation in the Management of HIV-Related Polyneuropathy". Pain Physician. 18 (4): E643–650. ISSN 2150-1149. PMID 26218955.
- ^ Deer, Timothy R.; Mekhail, Nagy; Provenzano, David; Pope, Jason; Krames, Elliot; Leong, Michael; Levy, Robert M.; Abejon, David; Buchser, Eric (August 2014). "The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee". Neuromodulation: Journal of the International Neuromodulation Society. 17 (6): 515–550, discussion 550. doi:10.1111/ner.12208. ISSN 1525-1403. PMID 25112889.
- ^ Hayek, Salim M.; Veizi, Elias; Hanes, Michael (October 2015). "Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience From an Academic Center Database". Neuromodulation: Journal of the International Neuromodulation Society. 18 (7): 603–608, discussion 608–609. doi:10.1111/ner.12312. ISSN 1525-1403. PMID 26053499.
- ^ Eldabe, Sam; Buchser, Eric; Duarte, Rui V. (2016-02-01). "Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature". Pain Medicine. 17 (2): 325–336. doi:10.1093/pm/pnv025. ISSN 1526-2375.
- ^ a b Kumar, Krishna; Buchser, Eric; Linderoth, Bengt; Meglio, Mario; Van Buyten, Jean-Pierre (January 2007). "Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts". Neuromodulation: Journal of the International Neuromodulation Society. 10 (1): 24–33. doi:10.1111/j.1525-1403.2007.00084.x. ISSN 1094-7159. PMID 22151809.
- ^ a b c d e f g h i j Cite error: The named reference
Kunnumourathwas invoked but never defined (see the help page). - ^ a b c d Dilorenzo, D. J.; and Bronzino, J. D. (2008). Neuroengineering. CRC Press. Chapter 7. ISBN 978-0-8493-8174-4
- ^ Melzack, R., and Wall, P.D. (1965). Pain mechanisms: a new theory" Science 150:971–979.
- ^ Shealy, C.N., Mortimer, J.T., and Resnick, J. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary reports. J. Int. Anesth. Res. Soc, 46:489–491, 1967.
- ^ Shimoji K, Higashi H, Kano T, Asai S, Morioka T. Electrical management of intractable pain. (1971) Masui (The Japanese journal of anesthesiology), 20: 444–447.
- ^ Kumar, K., Malik, S., and Demeria, D. (2002). "Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis" Neurosurgery 51(1), 106–115.
- ^ Bell, G. and North, R. (1997). "Cost-effectiveness analysis of spinal cord stimulation in treatment of failed back surgery syndrome". J. Pain Symptom Manage., 13 (5), 285–296.
- ^ Fuentes, R., Petersson, P., Siesser, W. B., Caron, M. G., & Nicolelis, M. A. L. (2009). Spinal Cord Stimulation Restores Locomotion in Animal Models of Parkinson's Disease" Science 323(5921), 1578-1582.
- ^ Spinal Cord Stimulator Sparks Hope for Parkinson's Disease – Duke University
- ^ Fénelon G, Goujon C, Gurruchaga JM, Cesaro P, Jarraya B, Palfi S, Lefaucheur JP. (2011). Spinal cord stimulation for chronic pain improved motor function in a patient with Parkinson's disease. Parkinsonism & Related Disorders (In Press)
- ^ Nam, Y., Brown, E.A., Ross, J.D., et al. (2009) "A retrofitted neural recording system with a novel stimulation IC to monitor early neural responses from a stimulating electrode". J Neurosci Methods. 178(1), 99–102.
