User:Mr. Ibrahem/Hydroxyzine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /haɪˈdrɒksɪziːn/ |
| Trade names | Atarax, Vistaril,[1] others |
| Other names | UCB-4492 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682866 |
| License data |
|
| Dependence liability | Very low[2] |
| Routes of administration | By mouth, intramuscular injection |
| Drug class | First generation antihistamine[3] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 93% |
| Metabolism | Liver |
| Metabolites | Cetirizine, others |
| Elimination half-life | Adults: 20.0 hours[4][5] Children: 7.1 hours[4] |
| Excretion | Urine, feces |
| Identifiers | |
| |
| Chemical and physical data | |
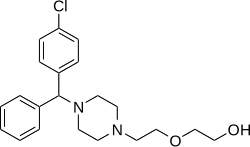
| Formula | C21H27ClN2O2 |
| Molar mass | 374.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hydroxyzine, sold under the brand names Atarax and others, is a medication of the antihistamine type.[6] It is used in the treatment of itchiness, anxiety, and nausea, including that due to motion sickness.[6] It; however, is less preferred to newer antihistamines such as cetirizine or loratadine for hives.[10] It is used either by mouth or injection into a muscle.[6]
Common side effects include sleepiness, headache, and a dry mouth.[6][7] Serious side effects may include QT prolongation.[7] It is unclear if use during pregnancy or breastfeeding is safe.[6] Hydroxyzine works by blocking the effects of histamine.[7] It is a first generation antihistamine in the piperazine family of chemicals.[6][3]
It was first made by Union Chimique Belge in 1956 and was approved for sale by Pfizer in the United States later that year.[6][11] In the United Kingdom 28 doses cost less than a pound.[7] In the United States the wholesale cost in 2018 was about 0.05 USD per dose.[12] In 2017, it was the 99th most commonly prescribed medication in the United States, with more than seven million prescriptions.[13][14]
References[edit]
- ^ "Drugs@FDA: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 2020-10-20. Retrieved 2020-08-05.
- ^ Hubbard, John R.; Martin, Peter R. (2001). Substance Abuse in the Mentally and Physically Disabled. CRC Press. p. 26. ISBN 9780824744977. Archived from the original on 2020-08-01. Retrieved 2019-08-20.
- ^ a b "Hydroxyzine". pubchem.ncbi.nlm.nih.gov. Archived from the original on 13 August 2020. Retrieved 4 March 2020.
- ^ a b Cite error: The named reference
pmid2866055was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid6141198was invoked but never defined (see the help page). - ^ a b c d e f g h i "Hydroxyzine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 27 March 2019. Retrieved 21 Nov 2018.
- ^ a b c d e British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. p. X. ISBN 978-0857112989.
- ^ "Hydroxyzine Use During Pregnancy". Drugs.com. 4 May 2020. Archived from the original on 30 October 2020. Retrieved 17 May 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 30 August 2020.
- ^ Fein, MN; Fischer, DA; O'Keefe, AW; Sussman, GL (2019). "CSACI position statement: Newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria". Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 15: 61. doi:10.1186/s13223-019-0375-9. PMID 31582993.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shorter E (2009). Before Prozac: the troubled history of mood disorders in psychiatry. Oxford [Oxfordshire]: Oxford University Press. ISBN 9780195368741. Archived from the original on 2020-01-02. Retrieved 2016-09-23.
- ^ "NADAC as of 2018-11-21". Centers for Medicare and Medicaid Services. Archived from the original on 2019-06-01. Retrieved 21 Nov 2018.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Hydroxyzine - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
