User:Mr. Ibrahem/Solriamfetol
 | |
| Clinical data | |
|---|---|
| Trade names | Sunosi |
| Other names | SKL-N05, ADX-N05, ARL-N05, YKP10A, R228060, and JZP-110; (R)-2-amino-3-phenylpropylcarbamate hydrochloride |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619040 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Norepinephrine–dopamine reuptake inhibitor[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~95%[1] |
| Protein binding | 13.3–19.4%[1] |
| Metabolism | Minimal[1] |
| Elimination half-life | ~7.1 hours[1] |
| Excretion | Urine (95% unchanged) |
| Identifiers | |
| |
| Chemical and physical data | |
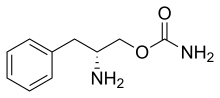
| Formula | C10H14N2O2 |
| Molar mass | 194.234 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Solriamfetol, sold under the brand name Sunosi, is a medication used to treat excessive sleepiness associated with narcolepsy and obstructive sleep apnea.[1] It is taken by mouth.[3]
Common side effects include headache, nausea, anxiety, and trouble sleeping.[1] Other side effects may include increased blood pressure and abuse.[3] It interacts with monoamine oxidase (MAO) inhibitors.[3] Safety in pregnancy and breastfeeding is unclear.[4] It is a norepinephrine–dopamine reuptake inhibitor (NDRI); though how it improves wakefulness is not entirely clear.[1][5]
Solriamfetol was approved for medical use in the United States in 2019 and Europe in 2020.[3][5] In the United States its costs about 690 USD per month as of 2021.[6] In the United States it is a Schedule IV controlled substance.[1]
References[edit]
- ^ a b c d e f g h i j k l "Sunosi- solriamfetol tablet, film coated". DailyMed. 16 October 2019. Archived from the original on 7 August 2020. Retrieved 24 November 2019.
- ^ "Sunosi EPAR". European Medicines Agency (EMA). 12 November 2019. Archived from the original on 8 November 2020. Retrieved 26 September 2020.
- ^ a b c d e "Solriamfetol Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 14 October 2021.
- ^ "Solriamfetol (Sunosi) Use During Pregnancy". Drugs.com. Archived from the original on 26 October 2020. Retrieved 14 October 2021.
- ^ a b "Sunosi". Archived from the original on 8 November 2020. Retrieved 14 October 2021.
- ^ "Sunosi Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 27 December 2021. Retrieved 14 October 2021.
